The beneficial effect of coronary collateral circulation (CC) in patients with ST-segment elevation myocardial infarction is controversial. The aim of this study was to evaluate the impact of CC before reperfusion with primary angioplasty (PA) on the long-term prognosis of these patients.
MethodsRetrospective observational study of a cohort of 947 patients treated with PA and TIMI grade ≤ 1 flow in a single center from 2005 to 2013. Propensity score matching was used to create 2 groups of 175 patients each, matched by the degree of CC (Rentrop 0-1 vs Rentrop 2-3). In the matched cohort, we determined the impact of CC on total mortality, cardiovascular mortality, and a combined adverse cardiovascular event endpoint for a median follow-up of 864 (interquartile range, 396-1271) days.
ResultsOf a total of 947 patients included, 735 (78%) had Rentrop 0 to 1 and 212 (22%) had Rentrop 2 to 3. During follow-up, 105 patients died, 71 from cardiovascular causes. In the matched cohort, the total mortality rate was similar between the 2 groups (Rentrop 0-1 [8.8%] vs Rentrop 2-3 [6.3%]; HR = 1.22; 95%CI, 0.50-2.94; P = .654). There were no differences in cardiovascular mortality (Rentrop 0-1 [4.6%] vs Rentrop 2-3 [2.3%]; sHR = 0.49; 95%CI, 0.14-1.62; P = .244) or the composite endpoint including cardiovascular death, reinfarction, target vessel revascularization, and coronary artery bypass surgery (Rentrop 0-1 [18.8%] vs Rentrop 2-3 [13.1%]; sHR = 0.68; 95%CI, 0.40-1.15; P = .157).
ConclusionsIn this contemporary series, the presence of good CC before PA was not associated with better long-term clinical outcomes.
Keywords
Coronary collateral circulation (CC) refers to the network of vascular channels that develops to bridge a severe coronary stenosis or connect myocardial territories supplied by different epicardial arteries.1 There are 2 main types of collateral vessels: capillary size collaterals, which are predominantly located in the endocardium, and large, muscular collaterals, which develop from preexisting arterioles in the epicardium.2
In the last few decades, the CC of the heart has been the subject of extensive research. Data derived from meta-analyses have shown that patients with ischemic heart disease and good CC (GCC) have a 36% lower risk of death than those with a poor or absent CC (ACC). However, this benefit is essentially seen in patients with stable chronic coronary disease and not in those with acute myocardial infarction.3
In patients with ST-segment elevation acute myocardial infarction (STEMI), the presence of GCC before reperfusion leads to a decrease in the size of the infarct and microvascular injury in the short-term, and prevents adverse ventricular modeling.4–7 Nonetheless, the impact of GCC on the long-term prognosis remains controversial, with contradictory results in the 2 small observational studies in this line.8,9
The aim of this study was to evaluate the impact of CC status determined before reperfusion on the long-term prognosis of STEMI patients treated with primary angioplasty (PA).
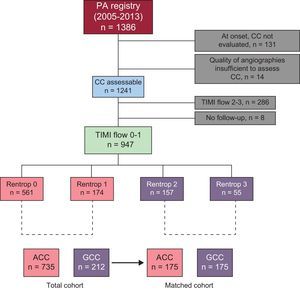
METHODSPatients and procedureThis retrospective cohort study was based on a local registry that included all consecutive patients with STEMI of less than 12 hours’ duration treated by PA in our center between January 2005 and December 2013. Approximately 85% of our STEMI patients have an indication for PA.10 The following patients were excluded from the study: those with documented Thrombolysis In Myocardial Infarction (TIMI) grade 2 or 3 anterograde flow in the artery causing the infarction prior to PA, those undergoing coronary angiography of a noncausal artery following PA, and patients whose CC could not be properly evaluated due to technical reasons (eg, absence of runs long enough to estimate the CC) or who were not available for follow-up after the procedure (Figure 1). The study, which was approved by the ethics committee of our hospital, was conducted in accordance with the principles set down in the Declaration of Helsinki.
All patients received dual antiplatelet therapy with a loading dose of 300mg aspirin and 600mg of clopidogrel. Since 2011 and according to their profile, patients received a 60-mg dose of prasugrel or 180mg of ticagrelor. An initial 5000-IU dose of sodium heparin was administered during the procedure. Additional bolus administration of heparin and glycoprotein IIb/IIIa inhibitors was given at the discretion of the attending interventional cardiologist. At completion of PA, patients were managed according to the recommendations of clinical practice guidelines.11 Dual antiplatelet therapy for 12 months was prescribed in all patients.
The patients’ demographic data and information on the procedure and follow-up were prospectively recorded in a database. Two experienced cardiologists retrospectively evaluated the angiographies in a blinded manner to classify the CC into grades. Disagreement between these assessments was resolved by a third interventional cardiologist. The CC to the culprit artery was graded according Rentrop's classification: grade 0, no filling of any collateral vessels; grade 1, filling of side branches of the culprit epicardial artery by collateral vessels; grade 2, partial filling of the culprit artery by collateral vessels; grade 3, complete filling of the culprit epicardial artery by collateral vessels.12 Patients were then divided into 2 groups according to their CC grade: the ACC group (Rentrop 0-1) and the GCC group (Rentrop 2-3). The Kappa index of agreement was used to evaluate the intraobserver and interobserver variability for the classification of patients into one group or the other in a single random sample of 100 patients.
Aims and Follow-upThe primary aim of the study was to assess the impact of the CC on all-cause mortality. The secondary aims were the cardiovascular mortality rate and a composite endpoint of cardiovascular events including cardiovascular death, nonfatal reinfarction, target vessel revascularization, and coronary artery bypass surgery.
The cause of death was assigned by 2 cardiologists responsible for the registry. If there was a discrepancy between the 2 cardiologists, a third was consulted. When the cause of death was lacking or there was no consensus, the patient was included in the group “cause of death unknown or unclassifiable”.
Reinfarction was defined as recurrent chest pain with ST-segment or T-wave changes and a new elevation of myocardial necrosis markers. Revascularization of the target vessel was defined as the need for angioplasty with or without stent placement due to restenosis or thrombosis. In patients with more than 1 event, only the first was used for the combined endpoint of cardiovascular events.
To complete the patient follow-up (initiated on the day of hospital admission) and to determine the clinical events, we used the electronic medical records and in some cases, telephone contact. The patients’ clinical history is managed by the SELENE program in our hospital and the HORUS platform for processes in other hospitals and in primary care centers.
Statistical AnalysisThe complete cohort participated in the first phase of the analysis. Qualitative variables are expressed as the frequency and percentage. The comparison of percentages between the groups was carried out with the chi-square test or Fisher exact test. Quantitative variables are expressed as the mean ± standard deviation or the median [interquartile range]. Mean values were compared using the Student t test, and median values with the nonparametric Mann Whitney U test. Because of the nonrandomized nature of the study and the multiple sources of bias that could have an influence on the prognostic effect of the CC in this scenario, we performed an analysis including propensity score matching to minimize the potential bias implied by investigating the effect of GCC within an observational study.13 To this end, we estimated the propensity to have ACC or GCC by logistic regression analysis including the following covariates: age, sex, body mass index, diabetes mellitus, smoking habit, hypertension, dyslipidemia, previous ischemic heart disease, multivessel disease, infarct in an anterior location, presence of stent thrombosis, and the pain-to-needle time (time from the onset of symptoms to the start of the procedure). The balance of these covariates was evaluated using an algorithm that generated blocks using the propensity score information and determined differences within each block with the Student t test for each of the covariates that participated in the propensity score estimate.14
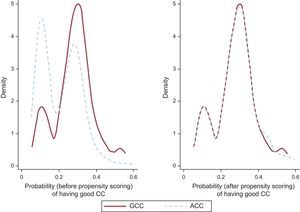
For the propensity score matching, we used a 1:1 protocol without replacement, with a caliper width equal to 0.2 of the standard deviation of the logit of the estimated propensity score.15 Two groups were created, matched by their propensity to have ACC or GCC. The balance between the covariates was then evaluated by obtaining the standardized differences of their means before and after matching. The predictive capacity of the model used to generate the propensity score was 0.69 (95% confidence interval [95%CI] 0.64-0.73). The density distribution of the propensity score before and after matching is shown in Figure 2.
Survival analysis was carried out in the matched cohort. For all-cause mortality, the survival curve of each group was estimated using the Kaplan-Meier method and compared using a stratified log-rank test. The Cox model was adjusted to the matching nature of the stratified sample by the pairs created.16,17 As death by other causes would act as a competing event, we used a regression model based on the Fine and Gray method for competing risks to analyze cardiovascular mortality and the composite endpoint of cardiovascular events.18 The cumulative incidence function was represented for each event.
Statistical analyses were carried out using SPSS 20.0 and STATA/IC 14.1. All tests were 2-tailed and results were considered statistically significant at a P value of<.05.
RESULTSOf the 947 patients included with TIMI 0-I flow in the artery responsible for the infarct, 735 (78%) had ACC, whereas 212 (22%) had GCC (Figure 1). The Kappa indices of agreement for interobserver and intraobserver variability were excellent (¿=0.95; 95% CI, 0.88-1.00, and ¿=0.97; 95% CI, 0.92-1.00, respectively).
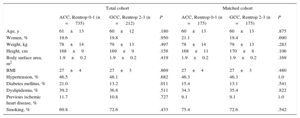
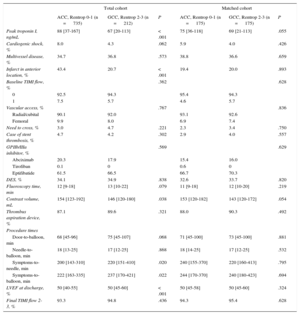
Baseline characteristics and procedure dataAnalysis of the baseline and demographic data showed that patients with GCC had a lower prevalence of diabetes mellitus than those with ACC (13.2% vs 21.0%; P=.011). Furthermore, the GCC group showed considerable differences relative to those with ACC regarding the procedure and clinical outcome (Table 1 and Table 2): a longer time between symptom onset and start of catheterization (symptoms-to-needle, 220 vs 200min; P=.020), longer time to revascularization of the occluded artery (symptoms-to-balloon, 237 vs 222min; P=.022), less contrast agent used in the procedure (146 vs 154mL; P=.038), fewer infarcts in an anterior location (20.7% vs 43.4%; P<.001), a lower troponin I peak value (67 vs 88 ng/mL; P<.001), and a higher left ventricular ejection fraction at discharge (51% vs 48%; P<.001).
Baseline Characteristics and Demographic Data Stratified by the Collateral Circulation Grade in the Overall Cohort and Matched Cohort
| Total cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|
| ACC, Rentrop 0-1 (n = 735) | GCC, Rentrop 2-3 (n = 212) | P | ACC, Rentrop 0-1 (n = 175) | GCC, Rentrop 2-3 (n = 175) | P | |
| Age, y | 61±13 | 60±12 | .180 | 60±13 | 60±13 | .875 |
| Women, % | 19.6 | 19.8 | .950 | 21.1 | 19.4 | .690 |
| Weight, kg | 78±14 | 79±13 | .497 | 78±14 | 79±13 | .283 |
| Height, cm | 168±9 | 169±9 | .158 | 168±11 | 170±8 | .106 |
| Body surface area, m2 | 1.9±0.2 | 1.9±0.2 | .419 | 1.9±0.2 | 1.9±0.2 | .169 |
| BMI | 27±4 | 27±3 | .869 | 27±4 | 27±3 | .480 |
| Hypertension, % | 46.5 | 48.1 | .682 | 46.3 | 46.3 | 1.0 |
| Diabetes mellitus, % | 21.0 | 13.2 | .011 | 15.4 | 13.1 | .541 |
| Dyslipidemia, % | 39.2 | 36.8 | .511 | 34.3 | 35.4 | .822 |
| Previous ischemic heart disease, % | 11.7 | 10.8 | .727 | 9.1 | 9.1 | 1.0 |
| Smoking, % | 69.8 | 72.6 | .433 | 75.4 | 72.6 | .542 |
ACC, poor or absent collateral circulation; BMI, body mass index; GCC, good collateral circulation.
Clinical Characteristics and Procedural Data Stratified by Collateral Circulation Grade in the Overall and Matched Cohort
| Total cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|
| ACC, Rentrop 0-1 (n = 735) | GCC, Rentrop 2-3 (n = 212) | P | ACC, Rentrop 0-1 (n = 175) | GCC, Rentrop 2-3 (n = 175) | P | |
| Peak troponin I, ng/mL | 88 [37-167] | 67 [20-113] | < .001 | 75 [36-118] | 69 [21-113] | .055 |
| Cardiogenic shock, % | 8.0 | 4.3 | .062 | 5.9 | 4.0 | .426 |
| Multivessel disease, % | 34.7 | 36.8 | .573 | 38.8 | 36.6 | .659 |
| Infarct in anterior location, % | 43.4 | 20.7 | < .001 | 19.4 | 20.0 | .893 |
| Baseline TIMI flow, % | .362 | .628 | ||||
| 0 | 92.5 | 94.3 | 95.4 | 94.3 | ||
| 1 | 7.5 | 5.7 | 4.6 | 5.7 | ||
| Vascular access, % | .767 | .836 | ||||
| Radial/cubital | 90.1 | 92.0 | 93.1 | 92.6 | ||
| Femoral | 9.9 | 8.0 | 6.9 | 7.4 | ||
| Need to cross, % | 3.0 | 4.7 | .221 | 2.3 | 3.4 | .750 |
| Case of stent thrombosis, % | 4.7 | 4.2 | .302 | 2.9 | 4.0 | .557 |
| GPIIb/IIIa inhibitor, % | .569 | .629 | ||||
| Abciximab | 20.3 | 17.9 | 15.4 | 16.0 | ||
| Tirofiban | 0.1 | 0 | 0.6 | 0 | ||
| Eptifibatide | 61.5 | 66.5 | 66.7 | 70.3 | ||
| DES, % | 34.1 | 34.9 | .838 | 32.6 | 33.7 | .820 |
| Fluoroscopy time, min | 12 [9-18] | 13 [10-22] | .079 | 11 [9-18] | 12 [10-20] | .219 |
| Contrast volume, mL | 154 [123-192] | 146 [120-180] | .038 | 153 [120-182] | 143 [120-172] | .054 |
| Thrombus aspiration device, % | 87.1 | 89.6 | .321 | 88.0 | 90.3 | .492 |
| Procedure times | ||||||
| Door-to-balloon, min | 68 [45-96] | 75 [45-107] | .068 | 71 [45-100] | 73 [45-100] | .881 |
| Needle-to-balloon, min | 18 [13-25] | 17 [12-25] | .868 | 18 [14-25] | 17 [12-25] | .532 |
| Symptoms-to-needle, min | 200 [143-310] | 220 [151-410] | .020 | 240 [155-370] | 220 [160-413] | .795 |
| Symptoms-to-balloon, min | 222 [163-335] | 237 [170-421] | .022 | 244 [170-370] | 240 [180-423] | .694 |
| LVEF at discharge, % | 50 [40-55] | 50 [45-60] | < .001 | 50 [45-58] | 50 [45-60] | .324 |
| Final TIMI flow 2-3, % | 93.3 | 94.8 | .436 | 94.3 | 95.4 | .628 |
ACC, poor or absent collateral circulation; DES, drug-eluting stent; GCC, good collateral circulation; GPIIb/IIIa, glycoprotein IIb/IIIa; LVEF, left ventricular ejection fraction; TIMI, Thrombolysis in Myocardial Infarction.
Values are expressed as the percentage or median [interquartile range], unless otherwise specified.
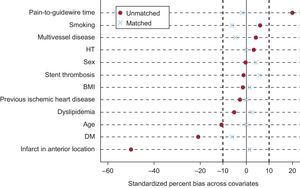
Adjustment according to the propensity score placed 175 patients in the GCC group, which were matched with 175 in the ACC group. The balance in the distribution of baseline covariates and procedure-related covariates between the properly matched patients is summarized in Table 1 and Table 2. The standardized differences of the means before and after matching are shown in Figure 3.
Events During Follow-upAfter a median follow-up of 864 [396-1.271] days, 105 patients in the overall cohort had died, 91 (12.4%) in the ACC group vs 14 (6.6%) in the GCC group. In the matched cohort, the primary event rate (all-cause mortality) was found to be similar in the 2 groups (ACC vs GCC, 8.8% vs 6.3%; hazard ratio [HR], 1.22; 95% CI, 0.50-2.94; P=.654) (Figure 4A).
A: Survival curves for all-cause mortality by subgroups according to collateral circulation grade. B: Cumulative incidence curves for the composite endpoint of cardiovascular events by subgroups according to collateral circulation grade. Model adjusted for mortality during follow-up as a competing event. 95% CI, 95% confidence interval; ACC, poor or absent collateral circulation; CABG: coronary artery bypass grafting; CVD, cardiovascular death; GCC, good collateral circulation; HR, hazard ratio; sHR: subhazard ratio; TVR, target vessel revascularization.
Among the 105 patients who died, 71 deaths were due to a cardiovascular cause, 66 (9.0%) in those with ACC and 5 (2.4%) in those with GCC. In the postmatching analysis, although the percentage of events in the ACC group doubled that recorded in GCC, the differences were not statistically significant (4.6% vs 2.3%; sub-HR [sHR], 0.49; 95% CI, 0.14-1.62; P=.244).
Finally, in the overall cohort, the rate of combined cardiovascular events (cardiovascular death, reinfarction, target vessel revascularization, and coronary artery bypass surgery) was 19.9% in the ACC group vs 13.7% in GCC. As occurred with cardiovascular mortality, in the matched cohort, the differences benefitting patients with GCC did not reach statistical significance (18.8% vs 13.1%; sHR, 0.68; 95% CI, 0.40-1.15; P=.157) (Figure 4B).
DISCUSSIONThis is the largest study to date evaluating the impact of CC status prior to PA reperfusion on the long-term prognosis of STEMI patients. The following are the main findings: 1) The presence of GCC before reperfusion treatment is uncommon in STEMI patients and is more frequent in infarcts affecting territories other than an anterior location, and 2) In the analysis of patients matched by propensity score, the presence of GCC before PA was not associated with a more favorable long-term outcome.
Collateral Circulation and Clinical EventsThe clinical relevance of CC in the era of coronary revascularization is controversial. Data derived from meta-analyses support a beneficial impact of CC on survival, mainly in patients with stable ischemic heart disease.3,19 However, in the setting of acute disease, where the incidence of new cardiovascular events remains high following the acute coronary syndrome,20 the results of the related studies are discordant. In Spain, the incidence of acute myocardial infarction in individuals older than 50 years is estimated at 90 000 cases per year.21
A recent study including more than 5000 participants evaluated associations between CC and clinical events in patients with a non–ST-segment elevation acute myocardial infarction. No associations were found between the CC grade and clinical events, such as death, nonfatal infarction, or revascularization of the target vessel.22
In patients with STEMI, the presence of CC leads to a lowering of the myocardial ischemia burden in the acute phase23 and a decrease in ventricular arrhythmia due to a reduction in the ischemia-mediated prolongation of the QT interval.24 Furthermore, CC can result in a reduction in the size of the infarct and microvascular injury, as well as adverse ventricular modeling in these patients.4–7 Several authors have evaluated the clinical impact of CC according to this pathophysiologic basis, with discrepant results. One of the first studies, which only included patients with anterior infarcts, found lower in-hospital mortality in those who exhibited some CC development (Rentrop 1-3) through a decrease in cardiogenic shock.25 Despite these findings, several other authors have reported no clinical benefits from CC.4,26,27 Various factors may explain these discrepancies: differences in the inclusion criteria (eg, location of the infarct, reperfusion methods used, and time from symptom onset to reperfusion), insufficient statistical power to evaluate “hard” clinical outcomes, a generally short follow-up, and a lack of statistical adjustment for potential confounders, a limitation seen in most of the published studies on this subject. The use of propensity scoring in the present study has enabled us to minimize in great part the differences between patients with different grades of CC. It should also be noted that the results and conclusions of this study were obtained in 2 patient subgroups that allowed matching. In this cohort, there were some notable differences between the groups studied, some of which have been described in previous articles. The most important from the clinical viewpoint were the smaller percentage of diabetic patients and the longer ischemia time in the GCC group, and differences in the artery responsible for infarction between patients who developed CC and those who did not. The percentage of infarcts affecting anterior territories in our series was significantly lower in patients who developed GCC (20.7% vs 43.4%; P<.001). This concurs with data from the available studies, in which the percentage of anterior infarcts in patients with GCC ranges from 14% to 45%.4,8,9,26,27
With regard to the follow-up time, only 2 studies have evaluated the long-term impact of CC, and their results differ.8,9 In the first, the presence of GCC was associated with longer survival and a lower rate of cardiovascular events, particularly in patients with more than 6hours since the onset of symptoms. The second study found no correlation between the CC grade before reperfusion with PA and the long-term prognosis in STEMI patients. In addition to the lack of statistical adjustment, the main limitation of both studies was the size of the sample included (235 and 330 patients, respectively). The present study, which has a clinical follow-up of more than 2 years, is the largest to date investigating this subject.
In summary, the results of this study show that the presence of GCC before PA treatment does not have an impact on the long-term prognosis of STEMI patients. This indicates that although a well-developed CC may attenuate ischemia and lead to a decreased size of the infarct in the earliest phase, it may also be a marker of chronic ischemia risk and more advanced heart disease, which could imply a poorer long-term prognosis. Precise evaluation of the time factor would likely be of considerable interest. That is, the time when CC developed in each patient: either chronically in patients with previous ischemia or immediately after the acute vessel occlusion. This aspect would be key to understanding the significance of CC in each case.
LimitationsOne of the main limitations of this study was the method used to evaluate CC, which was done using angiography. Other, more invasive methods are available, measuring intracoronary pressure or Doppler flow indices, and these more reliable and quantifiable.28 Nonetheless, they are not practical in the urgent scenario of PA. Furthermore, CC evaluation was not done in an independent core laboratory. Despite these limitations, the indices of agreement for classifying patients into each group (ACC or GCC) were excellent.
Another limitation of the study lies in the baseline differences between the groups studied: a higher percentage of diabetic patients, lower troponin I peak values, fewer infarcts in an anterior location, smaller contrast volume used, longer symptoms-to-balloon time, and better left ventricular ejection fraction at discharge in patients with GCC. The use of statistical techniques such as the propensity score would reduce these sources of bias in great part. However, other methodological limitations should be considered, such as variables that were not recorded (eg, the different antiplatelet regimens used and data on treatment during follow-up) and the reduction in sample size inherent to the statistical technique. In light of the differences described, stratification of the results according to the artery responsible for the infarction would have had considerable interest. However, as the analysis was performed using propensity scores in a smaller subgroup of patients, the dearth of events and subsequent lack of statistical power might have rendered the model inconsistent, and the results impossible to evaluate
Of note, since 2005, 9% of patients in our series underwent PA directly, without previous angiography of the contralateral vessel. These patients were excluded from the analysis because it was impossible to assess their CC (Figure 1).
CONCLUSIONSThe results of this study, which contains the largest number of patients reported to date, show that the presence of a well-developed CC in STEMI patients before PA reperfusion is not associated with a long-term decrease in all-cause mortality, cardiovascular mortality, or the incidence of a composite endpoint of cardiovascular events. These findings suggest that we should not neglect follow-up in these patients or fail to provide strong secondary prevention measures, as indicated for all patients with ischemic heart disease.
CONFLICTS OF INTERESTNone declared.
- –
In recent years, coronary CC has been the subject of extensive research. Data from meta-analyses point to a beneficial effect of the CC on patient survival, mainly in those with stable ischemic heart disease.
- –
It is uncertain whether coronary CC has a favorable effect in patients with an ST-segment elevation acute coronary syndrome.
- –
Only 2 observational studies with small samples have evaluated the impact of the CC on the long-term prognosis of these patients, with contradictory results.
- –
This study is the largest to date assessing the impact of the coronary CC on clinical events, and the follow-up period is longer than 2 years.
- –
It is the only study in this context that has used a propensity score statistical approach to adjust for confounding variables.
- –
The results reported may serve as a lead point for future research on the subject of CC in acute ischemic heart disease. Collateral circulation: Is it protective or a risk marker?