A new acute severe respiratory distress syndrome was first described in December 2019 in China and a novel coronavirus was soon identified afterward as being responsible for what is now known as coronavirus disease 2019 (COVID-19).1,2 In a short period of time, this pandemic has tested the capabilities of health care systems worldwide and led to the implementation of various confinement measures intended to stop the spread of the virus. In this setting, it has become essential to organize health care to provide medical attention to this new disease while maintaining appropriate clinical follow-up for other patients.
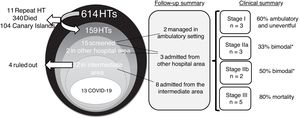
We present our experience with heart transplant (HT) recipient care during the pandemic in Madrid (Spain). Our program has performed 614 HT procedures in the last 30 years, with patients from a wide geographical area (including the Canary Islands) regularly followed up both at our hospital (as a referral center) and at their local institutions. All information was collected from our medical records (figure 1).
During a 2-month period (February 28 to April 28, 2020), all HT recipients were screened for COVID-19 symptoms. All recipients received information about the confinement and protective measures (face mask and hand hygiene) and about the procedure to be followed in the case of suspected COVID-19. We scheduled telephone follow-up for high-risk patients (ie, late allograft dysfunction) and deferred the visits of stable patients. We maintained visits for the most recent HT recipients (within 3 months after transplantation) in a protective circuit for outpatient care in our hospital. A coronavirus-free area called an intermediate area (IA) was established in our center to evaluate high-risk patients (solid organ transplant recipients, ventricular assist device patients, and oncologic patients) who needed evaluation due to suspected COVID-19. This IA comprised individual rooms that permitted patient evaluation, including chest X-ray and the collection of upper respiratory tract samples for coronavirus RNA detection by polymerase chain reaction (PCR) and blood samples. This IA was available 24hours a day, 7 days a week and eliminated the need for these patients to visit the emergency department. All IA staff received intensive training in procedures concerning patient isolation, individual protective equipment management, and routine sterilization. The clinical stage at evaluation according to a recently proposed classification3 is shown in figure 1. Stage I (mild) is the initial symptomatic phase, stage II (moderate) has pulmonary involvement without (IIa) and with (IIb) hypoxia due to local lung inflammation, and stage III (severe) is a systemic hyperinflammation syndrome.
Telephone follow-up was performed for 159 HT recipients. Two patients were admitted to a different institution (and required telephone consultation). We identified 15 HT recipients with a clinical picture that suggested COVID-19 and scheduled telephone calls twice a week. Of the 15, 2 were followed up by telephone in their regular residence and 13 needed complete evaluation in the hospital (12 in the IA and 1 in a different institution). Of the 12 HT recipients evaluated in the IA, 4 were sent home safely after COVID-19 was ruled out. None developed the infection in the subsequent 30 days. The remaining 8 patients tested positive and required hospitalization for monitoring and treatment.
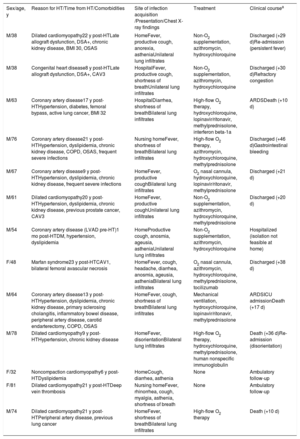
COVID-19 was diagnosed in 13 of the 159 patients screened (8.2%; 95% confidence interval, 4-12). Their clinical characteristics are described in table 1. Four of these patients have previously been reported.4 Notably, 15% of the patients had leucopenia in the preceding 3 to 6 months, as described previously.5 Nine (69%) of them received triple immunosuppressive therapy with either tacrolimus (n=7) or cyclosporine (n=6). None was receiving mTOR inhibitor therapy. Twelve of the patients were SARS-CoV-2 PCR-positive; the remaining patient was clinically diagnosed. In 4 patients (31%), the PCR test had to be repeated after a negative test result was obtained despite high clinical suspicion (1 patient provided 2 nasopharyngeal and 2 sputum samples before a positive result was obtained). We observed mild troponin elevation in 6 patients that was three times the cutoff value but it was not related to clinical events. Two patients developed severe leucopenia during follow-up that required granulocyte-colony stimulating factor.
Characteristics of HT recipients with COVID-19
| Sex/age, y | Reason for HT/Time from HT/Comorbidities | Site of infection acquisition /Presentation/Chest X-ray findings | Treatment | Clinical coursea |
|---|---|---|---|---|
| M/38 | Dilated cardiomyopathy22 y post-HTLate allograft dysfunction, DSA+, chronic kidney disease, BMI 30, OSAS | HomeFever, productive cough, anorexia, astheniaUnilateral lung infiltrates | Non-O2 supplementation, azithromycin, hydroxychloroquine | Discharged (+29 d)Re-admission (persistent fever) |
| M/38 | Congenital heart disease8 y post-HTLate allograft dysfunction, DSA+, CAV3 | HospitalFever, productive cough, shortness of breathUnilateral lung infiltrates | Non-O2 supplementation, azithromycin, hydroxychloroquine | Discharged (+30 d)Refractory congestion |
| M/63 | Coronary artery disease17 y post-HTHypertension, diabetes, femoral bypass, active lung cancer, BMI 32 | HospitalDiarrhea, shortness of breathBilateral lung infiltrates | High-flow O2 therapy, hydroxychloroquine, lopinavir/ritonavir, methylprednisolone, interferon beta-1a | ARDSDeath (+10 d) |
| M/76 | Coronary artery disease21 y post-HTHypertension, dyslipidemia, chronic kidney disease, COPD, OSAS, frequent severe infections | Nursing homeFever, shortness of breathBilateral lung infiltrates | High-flow O2 therapy, azithromycin, hydroxychloroquine, methylprednisolone | Discharged (+46 d)Gastrointestinal bleeding |
| M/67 | Coronary artery disease9 y post-HTHypertension, dyslipidemia, chronic kidney disease, frequent severe infections | HomeFever, productive coughBilateral lung infiltrates | O2 nasal cannula, hydroxychloroquine, lopinavir/ritonavir, methylprednisolone | Discharged (+21 d) |
| M/61 | Dilated cardiomyopathy20 y post-HTHypertension, dyslipidemia, chronic kidney disease, previous prostate cancer, CAV3 | HomeFever, productive coughUnilateral lung infiltrates | Non-O2 supplementation, azithromycin, hydroxychloroquine, methylprednisolone | Discharged (+20 d) |
| M/54 | Coronary artery disease (LVAD pre-HT)1 mo post-HTDM, hypertension, dyslipidemia | HomeProductive cough, anosmia, ageusia, astheniaUnilateral lung infiltrates | Non-O2 supplementation, azithromycin, hydroxychloroquine | Hospitalized (isolation not feasible at home) |
| F/48 | Marfan syndrome23 y post-HTCAV1, bilateral femoral avascular necrosis | HomeFever, cough, headache, diarrhea, anosmia, ageusia, astheniaBilateral lung infiltrates | O2 nasal cannula, azithromycin, hydroxychloroquine, methylprednisolone, tocilizumab | Discharged (+38 d) |
| M/64 | Coronary artery disease13 y post-HTHypertension, dyslipidemia, chronic kidney disease, primary sclerosing cholangitis, inflammatory bowel disease, peripheral artery disease, carotid endarterectomy, COPD, OSAS | HomeFever, cough, shortness of breathBilateral lung infiltrates | Mechanical ventilation, hydroxychloroquine, lopinavir/ritonavir, methylprednisolone | ARDSICU admissionDeath (+17 d) |
| M/78 | Dilated cardiomyopathy9 y post-HTHypertension, chronic kidney disease | HomeFever, disorientationBilateral lung infiltrates | High-flow O2 therapy, hydroxychloroquine, methylprednisolone, human nonspecific immunoglobulin | Death (+36 d)Re-admission (disorientation) |
| F/32 | Noncompaction cardiomyopathy6 y post-HTDyslipidemia | HomeCough, diarrhea, asthenia | None | Ambulatory follow-up |
| F/81 | Dilated cardiomyopathy21 y post-HTDeep vein thrombosis | Nursing homeFever, rhinorrhea, cough, myalgia, asthenia, shortness of breath | None | Ambulatory follow-up |
| M/74 | Dilated cardiomyopathy21 y post-HTPeripheral artery disease, previous lung cancer | HomeFever, shortness of breathBilateral lung infiltrates | High-flow O2 therapy | Death (+10 d) |
ARDS, acute respiratory distress syndrome; BMI, body mass index; CAV, coronary allograft vasculopathy; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DSA, donor specific antibodies; F, female; HT, heart transplant; LVAD, left ventricular assist device; M, male; OSAS, obstructive sleep apnea syndrome; O2, oxygen.
The COVID-19 treatment was based on our institutional protocol, which was revised on a daily basis. We discontinued calcineurin inhibitors in 6 patients (in the 5 stage III patients and when lopinavir/ritonavir was prescribed in 1).
All 3 stage I patients remained uneventful. Three patients had a bimodal course with recurrence of symptoms and elevated inflammatory markers before definitive improvement was achieved. Four patients (31%) died; all had stage III disease at diagnosis.3 The causes of death were respiratory failure (2 patients), bacterial infection (1 patient), and hemorrhagic shock due to retroperitoneal hematoma attributable to low-molecular-weight heparin (1 patient).
The incidence of COVID-19 infection in our HT population was 8%, despite the indication of intensive protective measures for this high-risk population. This incidence is higher than that described in the Madrid area (0.9% in the general population with confirmed positive SARS-CoV-2 PCR6).
According to the results of the present study, the safe follow-up can be established of HT recipients during the COVID-19 pandemic. The implementation of an IA is useful for evaluating individuals with suspected COVID-19 while minimizing the risk of infection related to a hospital visit.
The mortality rate was high, although it may be explained by the patients’ high comorbidity and by the severity of the clinical presentation.
We should be aware of the high percentage of false-negative PCR tests in respiratory samples of the HT population that may hamper the diagnosis of atypical or mild cases.
FundingThis research was funded by the Centro de Investigación en Red de Enfermedades Cardiovasculares (CIBERCV), Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness. This work was supported by Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COV20/00181) — co-financed by the European Development Regional Fund, “A way to achieve Europe”.
We are grateful to Javier de Juan Bagudá and Jorge Nuche for critically revising the article.