Brugada syndrome (BS) is a channelopathy with an associated risk of malignant ventricular arrhythmias and sudden death. The diagnosis is established based on the patient's electrocardiography (ECG) pattern and clinical characteristics. The main treatment for BS is defibrillator implantation, although promising ablation techniques have been developed recently.1 In a large percentage of patients, the baseline ECG findings are normal or inconclusive, and a drug challenge is required to confirm the condition. This may also occur with members of the patients’ families, who can inherit the disease. Genetic study can identify the cause, confirm the diagnosis, and avoid unnecessary tests and follow-up in patients and their family members.
The main gene implicated in BS is the sodium channel gene, SNC5A, but other genes may make a smaller contribution. For a genetic study to be considered complete, in addition to examining all these genes, copy number variations (CNV), such as large deletions or duplications, should be ruled out, as a small percentage of BS cases may be due to these variants. We present the case of a family with BS, whose genetic study by massive next-generation sequencing (NGS) enabled identification of the etiology.
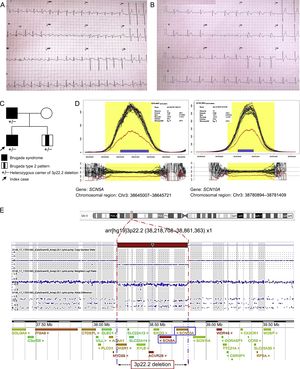
The proband, a 13-year-old boy, was hospitalized for palpitations after intense exercise. He had experienced no previous symptoms and was not receiving drug therapy. Before this event, he had undergone monitoring by the arrhythmia unit with yearly ECG testing because his father had been diagnosed with BS (showing a spontaneous type 1 ECG pattern and induced fibrillation on electrophysiology study) and was treated with defibrillator implantation. On admission, ECG testing showed an atrial flutter associated with a Brugada type 1 pattern (Figure A). The flutter remitted spontaneously, and a nodal rhythm with the same type 1 pattern was recorded later (Figure B). The patient was discharged in sinus rhythm, without the need for medication. He underwent an outpatient electrophysiology study, in which no ventricular arrhythmias were induced. Holter ECG testing showed sinus bradycardia, sinus pauses, and frequent atrial extrasystoles, findings considered to indicate probable sinus node dysfunction.
Familial Brugada syndrome. A. Electrocardiogram of the index case in atrial flutter. B. Posterior Brugada type 1 pattern. C. Family pedigree; familial genetic study (SNP-array). D. Analysis of NGS coverage shows a deletion in heterozygosis; abscissa axis, genomic region; ordinate axis, sequence coverage (number of reads); each black line represents 1 single case; the blue line, the median of all cases, and the red line, the index case. E. 3p22.2 deletion, characterized by SNP-array; the graph shows a signal decrease in this region. NGS, massive next-generation sequencing. This figure is shown in color only in the online version of the article.
The boy's father had never experienced shocks from the device, and had shown no signs of sinus dysfunction or atrial arrhythmias. Genetic study of the SCN5A gene had been performed previously by Sanger sequencing, with negative results. The proband's 11-year-old brother was asymptomatic and showed a Brugada type 2 pattern on the baseline ECG. When the proband was hospitalized, drug challenge had not been performed in the 2 boys. The father had no brothers. The mother was healthy/asymptomatic and her ECG was normal. There were no other known cases in the family (Figure C).
Genetic study of the boy using an NGS panel identified complete deletion of the SCN5A and SCN10A genes (Figure D). Confirmation using another, more specific molecular technique for CNV study (SNP-array) enabled characterization of the deletion (Figure E), which included 8 genes. Only 3 of these genes have been associated with the disease: SCN5A and SCN10A (BS), and ACVR2B (complex congenital heart defects). Genetic study of the family (by SNP-array, low-cost and accurate) identified the same deletion in the affected father and asymptomatic brother.
The deletion had not been described previously. A similar deletion is recorded in the DECIPHER database, but only 3 of the genes identified are involved (EXOG, SCN5A and SCN10A); it is considered pathogenic and was found in a male without a described phenotype. Several studies have reported large partial deletions in the SCN5A gene in BS patients.2–5 In all these cases, multiplex ligation-dependent probe amplification (MLPA) was used after a previous genetic study had yielded negative results (Table). Lastly, we mention the coexistence of atrial flutter and BS in the proband. SCN5A has an important pathophysiological role in sinus node automatism, and the mutations that generate hypofunction of this canal have been associated with both sinus dysfunction (with atrial arrhythmias) and BS.6
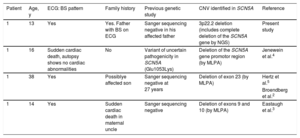
BS Patients With a Previously Negative or Inconclusive Genetic Study, Later Found to Have a Deletion in the SCN5A Gene
| Patient | Age, y | ECG: BS pattern | Family history | Previous genetic study | CNV identified in SCN5A | Reference |
|---|---|---|---|---|---|---|
| 1 | 13 | Yes | Yes. Father with BS on ECG | Sanger sequencing negative in his affected father | 3p22.2 deletion (includes complete deletion of the SCN5A gene by NGS) | Present study |
| 1 | 16 | Sudden cardiac death, autopsy shows no cardiac abnormalities | No | Variant of uncertain pathogenicity in SCN5A (Glu1053Lys) | Deletion of the SCN5A gene promotor region (by MLPA) | Jenewein et al.4 |
| 1 | 38 | Yes | Possiblye affected son | Sanger sequencing negative at 27 years | Deletion of exon 23 (by MLPA) | Hertz et al.5 |
| Broendberg et al.2 | ||||||
| 1 | 14 | Yes | Sudden cardiac death in maternal uncle | Sanger sequencing negative | Deletion of exons 9 and 10 (by MLPA) | Eastaugh et al.3 |
BS, Brugada syndrome; CNV, copy number variations; ECG, electrocardiogram; MLPA, multiplex ligation-dependent probe amplification; NGS, next-generation sequencing.
In conclusion, this is the first reported example of complete deletion of the SCN5A and SCN10A genes causing familial BS. It provides a clear illustration of the usefulness of NGS in the diagnosis of these diseases. It is now possible to carry out a complete genetic study using this technique, which enables identification of point variants and CNV in the genes of interest, avoids false-negative results, and provides adequate family screening. The development of this technology is leading to the identification of new molecular mechanisms and broadening our knowledge of the etiology of these diseases.
CONFLICTS OF INTERESTJ.P. Trujillo-Quintero, J.P. Ochoa and D. de Uña belong to the Clinical Department of Health in Code, a company with extensive experience in the genetic diagnosis of cardiovascular diseases.
We thank the family for placing their trust in the clinical team, and all members of the laboratory and bioinformatics areas, who are an essential part of this working group.