The objective was to describe the vegetation changes in patients with endocarditis and evaluate their prognostic importance during hospitalization. We selected patients with left-sided endocarditis and two transesophageal echocardiograms separated by at least 8 days. Patients who required surgery or died during the first week after diagnosis of the disease were excluded. Patients were classified into three groups: I, patients whose vegetation increased in size (n=34); II, patients with vegetations that did not vary in size (n=62); and III, patients whose vegetation decreased in size (n=59). Patients whose vegetation increased in size more frequently required surgery. Multivariate analysis showed that the increase in the vegetation is independently associated with increased mortality: adjusted odds ratio, 4.12 (95% confidence interval, 1.14-14.9; P=.031).
Keywords
The prognostic value of the size of the vegetation in patients with endocarditis and its “natural” course with medical treatment has not been studied. The latest guidelines1, 2 on endocarditis refer to these earlier studies,3, 4, 5 in which the persistence of vegetation, in the absence of valve dysfunction, was not associated with late complications.
The objective of this report is to describe the changes in vegetation size in left-sided endocarditis and to evaluate its clinical and prognostic importance during the patient's hospital stay.
MethodsWe designed a multipurpose cohort study and prospectively included patients admitted to the hospital with a definitive diagnosis of endocarditis according to the Duke criteria.6
Of all the episodes included, we selected the patients that met two criteria: a) left-sided endocarditis, and b) availability of two transesophageal echocardiograms (TEE) separated by at least 8 days, considering the first TEE as the diagnostic echocardiogram and the second as the last echocardiogram of the patient prior to hospital discharge, surgery or death.
Those patients who required emergency surgery or died during the first week after the diagnosis were excluded.
Of 683 episodes of endocarditis, 155 corresponding to 155 patients constituted our study group. Those patients whose first TEE did not reveal vegetation but whose second image did were included in the analysis.
To determine the degree of variability of the measurements, the observer was subjected to a reliability study, and an intraclass correlation coefficient of 0.965 (95% confidence interval [CI], 0.916-0.985) was obtained.
The difference between the diameters measured in the two TEE was determined and the patients were classified according to three groups: I, patients with endocarditis whose vegetation increased in size by 3mm or more (n=34); II, patients whose vegetation was similar in both TEE, considering this to be the case when the difference between the two measurements was less than 3mm (n=62); and III, patients whose vegetation decreased in size by 3mm or more (n=59).
ResultsIn the in-hospital follow-up of patients with endocarditis who did not require emergency surgery and did not die during the first week after the diagnosis, we observed that the vegetations increased in size in 21.9%, remained the same in 40% and diminished in 38.1%.
There were no significant differences in patient age, the existence of previous heart disease or the presence of comorbidity.
The most common clinical presentation consisted of fever and cardiac symptoms, with no significant differences. We found no differences in the etiologic distribution (Table 1), and the site of the infection was similar.
Table 1. Distribution of the Causative Microorganisms.
| Group I (n=34) | Group II (n=62) | Group III (n=59) | P | |
| Streptococcus bovis | 1 (2.9) | 2 (3.2) | 3 (5.1) | .846 |
| Streptococcus viridans | 5 (14.7) | 12 (19.4) | 6 (10.2) | .38 |
| Enterococcus | 2 (5.9) | 5 (8.1) | 4 (6.8) | .962 |
| Other streptococci | 1 (2.9) | 2 (3.2) | 4 (6.8) | .846 |
| Staphylococcus aureus | 5 (14.7) | 7 (11.3) | 10 (16.9) | .535 |
| Coagulase-negative Staphylococcus | 8 (23.5) | 12 (19.4) | 12 (20.3) | .95 |
| Gram-negative bacilli | 3 (8.8) | 6 (9.7) | 3 (5.1) | .591 |
| Fungi | 0 | 0 | 0 | |
| HACEK group | 1 (2.9) | 0 | 0 | .129 |
| Anaerobic bacteria | 0 | 2 (3.2) | 0 | .239 |
| Polymicrobial infection | 1 (2.9) | 4 (6.5) | 7 (11.9) | .307 |
| Others | 0 | 1 (1.6) | 4 (6.8) | .145 |
| Negative cultures | 7 (20.6) | 9 (14.5) | 6 (10.2) | .44 |
HACEK, Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae.
Data are expressed as n (%)
The mean interval between the two echocardiograms performed was: group I, 16 days (range: 12 to 33 days); group II, 24 days (14 to 37 days); and group III, 25 days (15 to 41 days) (P=.059).
The vegetation size in the baseline TEE was greatest in group III (group I, 0mm [range: 0 to 14mm]; group II, 8mm [2 to 14mm]; and group III, 14mm [10 to 19mm]). The size of the vegetations in the last TEE was greatest in the patients in group I (P=.001). The mean sizes were: group I, 14mm (range: 10 to 24mm); group II, 8mm (0 to 13mm); and group III, 4mm (0 to 9mm).
The patients had similar hospital stays: group I, 58±37 days; group II, 50±24 days; and group III, 55±25 days.
During the course of the study, we found no differences in the development of heart failure, embolism, shock, periannular complications or persistent infection (Table 2).
Table 2. Clinical Course of the Patients During Their Hospital Stay.
| Group I (n=34) | Group II (n=62) | Group III (n=59) | P | |
| Heart failure | 18 (52.9) | 31 (50) | 24 (40.7) | .222 |
| CNS embolism | 7 (20.6) | 14 (22.6) | 17 (28.8) | .607 |
| Hepatorenal axis embolism | 4 (11.8) | 7 (11.3) | 11 (18.6) | .46 |
| Renal failure | 9 (26.5) | 10 (16.1) | 17 (28.8) | .609 |
| Periannular complications | 9 (26.5) | 25 (40.3) | 16 (27.1) | .214 |
| AV block | 1 (2.9) | 9 (14.5) | 2 (3.4) | .036 |
| At least moderate valve failure | 25 (73.5) | 43 (69.4) | 41 (69.5) | .898 |
| Persistent infection | 12 (35.3) | 19 (30.6) | 24 (40.7) | .514 |
| Shock | 4 (11.8) | 4 (6.5) | 4 (6.8) | .609 |
| Surgery | 21 (61.8) | 27 (43.5) | 15 (25.4) | .002 |
| Death | 11 (32.4) | 15 (24.2) | 9 (15.3) | .053 |
AV, atrioventricular; CNS, central nervous system.
Data are expressed as n (%)
Of the 34 patients in group I, 21 required surgery and 5 of these patients died. There were 6 deaths among the patients who did not require surgery.
The need for surgery was greater among the patients whose vegetation increased in size. The mean time elapsed between diagnosis and surgery was similar: group I, 19 days (range: 14 to 46.5 days); group II, 25 days (13 to 47 days); and group III, 32 days (17 to 63 days) (P=.6).
The mortality rate was higher in the patients whose vegetation increased in size. We observed no differences in the causes of death; the most frequent cause was heart failure.
To evaluate the relationship between the increase in vegetation size during the in-hospital follow-up and the variable death, we adjusted logistic regression models. We included the clinically relevant variables, that is, heart failure at any time during the course of the study, central nervous system embolism, septic shock, signs of persistent infection, Staphylococcus aureus as the causative agent, changes in the vegetation, presence of periannular complications and need for surgery. Finally, we adjusted for all the variables initially included.
In our study, the increase in vegetation size was independently associated with a higher rate of mortality with respect to the group in which the vegetations decreased in size: adjusted odds ratio (aOR)=4.12 (95% CI, 1.14-14.9; P=.031). This was not the case in patients whose vegetation remained stable: aOR=2.07 (95% CI, 0.7-6.1; P=.186).
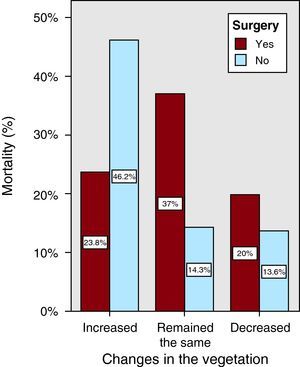
Given the greater need for surgery in the group of patients whose vegetations increased in size, we included in the model the interaction between surgery and growth of the vegetation in order to determine whether surgery modified the impact that the increment in the vegetation had on mortality. Each group was analyzed separately, and adjustment was made for the variables mentioned above. Among the patients with endocarditis who did not undergo surgery, the group with vegetations that increased in size had a higher mortality rate than the group in which the vegetations diminished: aOR=6.73 (95% CI, 1.37-33.12; P=.019). Among the patients who did undergo surgery, the mortality rate in group I was not higher than that of group III: aOR=1.65 (95% CI, 0.26-10.43; P=.595), nor was that of group II: aOR=3.7 (95% CI, 0.7-19.4; P=.124) (Figure 1).
Figure 1. Impact of the increment in the vegetation on mortality.
DiscussionDespite the advances in the diagnosis of endocarditis and the improvements in both the medical and surgical treatment, infective endocarditis mortality continues to be very high, around 16% (11% to 26%).1, 2, 7, 8, 9
In every episode of endocarditis, the clinical course is influenced by the sum of one or more risk markers present at the time of the diagnosis,10, 11 such as the baseline characteristics of the patient, the echocardiographic findings and microbiological factors.2, 10
Numerous studies have been carried out in the attempt to determine the importance of the presence and morphological characteristics of the vegetation in the prognosis of the endocarditis patient.7, 12 Some of these studies have shown that the size of the vegetation at the time of admission is associated with a higher risk of embolism and that the presence of large vegetations (>10mm) results in a poorer prognosis.7, 11, 12
Few studies have investigated the change in the vegetation size and its possible relationship to the prognosis of the patient.3, 4, 5 Our objective was to determine whether the increment in the diameter of the vegetation resulted in a poorer in-hospital prognosis.
To carry out this study, we excluded those patients who required emergency surgery or died during the first week after diagnosis. Therefore, the most seriously ill patients were excluded and, thus, our group of patients had a priori better prognoses than the overall population of patients with endocarditis.
Vuille et al3 concluded that the morphological changes in the vegetation during antibiotic therapy were not related to long-term prognosis.
Rohmann et al4 observed that the patients in whom there was an increase in the size of the vegetation had a more indolent clinical course. However, this study has two important limitations: the authors did not apply the Duke criteria and 50% of the blood cultures were negative. Moreover, only 3 patients died (3.6%); thus, there are reasonable doubts as to whether all the patients included had endocarditis.
In this study, we observed that, after adjusting for all the variables having the greatest relevance in the prognosis of the patient with left-sided endocarditis, the patients whose vegetations increased in size had a greater need for surgery and a higher mortality rate. This probably indicates the lack of local control of the infection; however, due to the low statistical power of the sample, we have not been able to find a significant association between the increment in the vegetation and a higher rate of heart disease or periannular complications. Moreover, surgery was seen to modify the effect of the increase in vegetation size on mortality.
These results indicate that the prognosis of the patient during the course of endocarditis depends not only on the initial size of the vegetation, but also on the changes it undergoes throughout the disease. Thus, the treatment of endocarditis should be guided not only on the basis of the clinical course, but also by certain echocardiographic data, such as the change in vegetation size. We recommend the systematic use of TEE in patient follow-up to assess the changes in the size of the vegetation and the development of other complications of this disease.
Conflicts of interestNon declared.
Received 14 September 2010
Accepted 17 October 2010
Corresponding author: Instituto Cardiovascular, Hospital Universitario San Carlos, Prof. Martín Lagos s/n, 28040 Madrid, Spain. ivilac@medynet.com