Provisional side-branch stenting is currently the most widely accepted percutaneous technique for the treatment of bifurcation lesions. However, abrupt closure of the side branch may occur after main vessel stent implantation. Resolving side-branch stenosis under these conditions may pose major technical difficulties. We describe a new technique to resolve uncrossable side-branch occlusion following main-vessel stent implantation during provisional side-branch stenting. The technique consists of using the jailed wire to dilate the occluded side branch. We first use a low profile, 1.25-mm diameter balloon catheter. A regular balloon is then inflated through the same wire to open the side branch, crushing the proximal part of the main vessel stent. At this point, a second stent is implanted at the side-branch, finishing the procedure as an inverted crush stenting. The described strategy may be useful in cases of uncrossable side-branch occlusion causing severe hemodynamic impairment that cannot be swiftly managed with conventional methods.
Keywords
Provisional side-branch (SB) stenting is currently the most widely accepted percutaneous technique for the treatment of bifurcation lesions.1 This approach basically consists of stenting the main vessel and balloon dilation of the SB. When the results are suboptimal at the SB, a second stent can be implanted in this coronary segment. Provisional stenting has been called a simple approach;2 however, the technique may not be so simple and, after main vessel stent implantation, abrupt closure of the SB may occur due to carina/plaque shifting. Resolving SB stenosis under these conditions may pose major technical difficulties, especially if the vessel closure causes severe hemodynamic impairment during the procedure.
MethodsWe describe a new technique to quickly resolve uncrossable SB occlusion after main vessel (MV) stent implantation during provisional SB stenting in 3 patients. It consists of using the jailed wire to dilate the occluded side branch. We first use a low profile. 1.25-mm diameter balloon catheter. A regular balloon is then inflated through the same wire to open the side branch, crushing the proximal part of the main vessel stent. At this point, a second stent is implanted at the side branch, finishing the procedure as an inverted crush stenting.
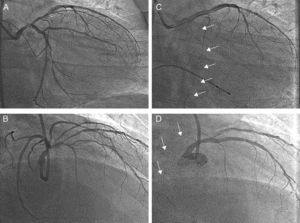
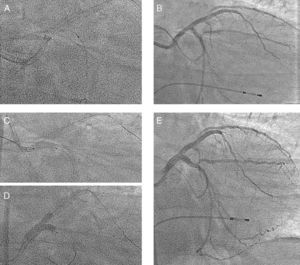
Results Case 1A 53-year-old male presented with symptoms of unstable angina. Coronary angiography was performed and showed left main (LM) artery and multivessel disease with normal ejection fraction. Figure 1A and B show baseline left coronary angiography. Two overlapping Everolimus-eluting stents were implanted (2.5×12 and 3×18mm) at mid left anterior descending (LAD) artery. A third long stent (3.5×23mm) was implanted at the LM-LAD, across the ostium of the circumflex (Cx) artery. The Cx artery became occluded while the jailed wire remained in place as a marker (Figure 1C and D). Hypotension and severe bradycardia developed, requiring dopamine and a provisional pacemaker. The clinical course was complicated by ventricular fibrillation, which was resolved by an electrical cardioversion. In this situation, the operators tried to recanalize the occluded artery using hydrophilic wires, over-dilation of the proximal part of the LM stent (4mm balloon), and a Venture wire control catheter. Despite the efforts of several operators, none of these maneuvers was successful. Finally, a 1.25×6mm low-profile balloon catheter was inserted through the jailed wire (Figure 2A) and passed without difficulty through the Cx origin. After a first dilation, a conventional balloon was inserted and inflated. At that point, the artery opened (Figure 2B), and arterial pressure was immediately restored. With the patient in more stable condition, a new Everolimus-eluting stent (3.5×18mm) was implanted in the Cx artery (Figure 2C), crushing the LM-LAD stent. The LAD artery was rewired, and the procedure was finished with a kissing balloon inflation (Figure 2D), obtaining an optimal immediate angiographic result (Figure 2E). The patient developed a non-Q wave myocardial infarction (peak CK=1612 IU/L). He was discharged after a few days, and at 15 months follow-up remains free of symptoms.
Figure 1. Patient 1. Coronary angiogram before stent implantation across the left main bifurcation (A and C), and after left main stent implantation (B and D). The circumflex artery was totally occluded and the jailed wire remained in place as a marker of its position (arrows). A and B: right anterior oblique 50, craneal 30. C and D: right anterior oblique 15, caudal 15.
Figure 2. Patient 1. A 1.25×6mm, low-profile balloon was inserted through the jailed wire (A). After dilation with a conventional balloon, the circumflex artery artery opened (B). A 3.5×18-mm stent was implanted at the circumflex artery (C), crushing the left main-left anterior descending artery stent. Kissing balloon inflation (D). Final angiographic result (E).
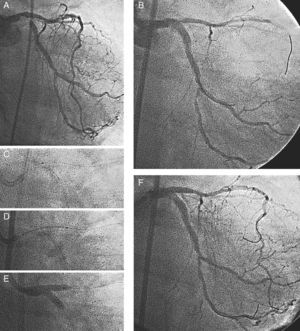
Case 2A 69-year-old female with resting angina, atrial fibrillation, and pulmonary edema was admitted to our hospital for cardiac catheterization. Left ventricular ejection fraction was 61%. Coronary angiography showed severe distal left main artery stenosis and significant lesions involving the mid-Cx and mid-RC arteries. The right anterior oblique (RAO) view (Figure 3A) showed severe stenosis at the distal LM bifurcation (Medina 1,0,1). A Paclitaxel-eluting stent (3.5×20mm) was implanted towards the Cx artery with an excellent immediate result at the distal LM artery and Cx ostium, but with severe compromise at the LAD origin (Figure 3B) and a reduction in coronary flow (TIMI II). At that time, we expanded the stent proximally with a 4.5-mm diameter balloon and attempted to rewire the LAD artery. A Whisper 0.014” wire, alone or supported by a Prowler microcatheter, was used for rewiring; however, a dissection occurred during this maneuver and the wire did not cross over into the true lumen, while flow in the vessel progressively worsened. In view of the patient's deteriorating status, the operators decided to use the jailed wire that had remained in the true lumen. We followed the same steps described in the previous case. First, a 1.25×6mm low-profile balloon catheter was inserted (Figure 3C), and the LAD ostium was then dilated with a conventional 2.5-mm diameter balloon catheter. A 3×20mm Paclitaxel-eluting stent was implanted, crushing the Cx stent (Figure 3D). Finally, the Cx artery was rewired and a kissing balloon inflation was performed (Figure 3E). An excellent angiographic result was obtained (Figure 3F). Mid-Cx and RCA were successfully treated in another procedure. Plasma CK levels after the procedure were normal. An angiographic reevaluation 13 months later showed persistence of the initial good result.
Figure 3. Patient 2. Coronary angiogram showing severe stenosis at the distal left main bifurcation {1,0,1} (A). Stent implantation (3.5×20mm) at left main-circumflex with severe compromise at the left anterior descending artery origin (B). A 1.25×6mm low-profile balloon catheter was inserted through the jailed wire (C). A 3×20-mm stent was implanted crushing the Circumflex stent (D). Kissing balloon inflation was performed (E). An excellent angiographic result was obtained (F).
Case 3A 60-year-old man with a previous inferior myocardial infarction and a current history of angina and dyspnea in functional class II-III was referred to our hospital for percutaneous revascularization. He had undergone a previous catheterization at another center, where LM and 3-vessel disease with poor left ventricular function were detected. The RC artery was chronically occluded with collateral flow from the Cx artery, and the left ventricular ejection fraction was 35% with posterobasal akinesia. After wiring the Cx artery, a Everolimus-eluting stent (3×12mm) was implanted across the LM bifurcation with proximal over-expansion to 4mm. Some compromise was observed at the Cx ostium, and the operators decided to rewire this vessel, leaving the jailed wire in place. During the wiring maneuver, the artery was dissected and later occluded, with disappearance of collateral flow to the RC artery. The patient developed hypotension and chest pain, and the operators decided to use the jailed wire (as previously described) to stent the spiral dissection of the Cx artery. After dilation with a 1.25×6mm low-profile balloon catheter and a 2.5mm regular balloon, the first stent (Everolimus-eluting stent, 3×18mm) was implanted at the Cx ostium, crushing the proximal part of the LM-LAD stent. Two additional overlapping stents (Everolimus-eluting stents, 3×12mm and 2.5×12mm) were then implanted, sealing the spiral dissection. We completed the procedure with a final kissing balloon inflation at the LAD-Cx. Plasma CK levels after the procedure were normal. The patient was discharged a few days later, and remains free of symptoms at 3 months follow-up.
DiscussionWhen one stent is implanted across a bifurcation, there is a risk of occlusion of the SB. Rewiring the occluded vessel may be difficult, especially if abrupt closure of the vessel causes clinical deterioration of the patient, requiring urgent reopening of the vessel (Figure 1). A dissection at the origin of the SB may further complicate the procedure after MV stenting. This complication may cause impairment of vessel flow and difficulties in accessing the true lumen. Thus, wiring the SB at the beginning of the procedure is mandatory. It allows predilation of the SB origin if necessary. Furthermore, once the main vessel stent is implanted, the jailed wire helps to keep the SB open. In case of SB occlusion, the wire is also a good marker of position (Figure 1, and Figure 3) and facilitates access to the SB by modifying the angle. Finally, the jailed wire facilitates deep intubation of the guiding catheter if firmer support is required to cross the SB ostium with the balloon.
In this article, we propose a simple technique: the use of the jailed wire to dilate a dissected/occluded uncrossable SB. In our patients with distal left main disease, once all of our technical armamentarium had failed, balloon dilation of the occluded SB through this wire turned out to be a life-saving maneuver. There are scarce data in the literature reflecting the percentage of cases when it is impossible to cross the SB after MV stent implantation. In our experience,3, 4 it was impossible to access the SB in 3%-8% of patients with bifurcated lesions treated with provisional SB stenting. New devices may be helpful in achieving SB access in complex bifurcation. Recently, some studies5, 6 have described the use of a catheter with a deflectable tip (Venture) as an option after the failure of conventional techniques in patients with different types of complex coronary bifurcation lesions, with a success rate of around 85%. This device requires experienced operators, and accessing the SB can sometimes be a lengthy process. In our first patient, the device failed to re-cross the occluded Cx artery. The complication described in our patients is not a frequent occurrence, and when it does occur, the operator is usually able to recross and reopen the occluded SB with currently available wires and balloons. However, in cases of provisional SB stenting in large proximal bifurcations complicated by uncrossable SB occlusion, this technique may prove to be a quick solution for patients in critical hemodynamic condition.
LimitationsOur series is small (as is the incidence of this complication), and our study is retrospective. Failure in passing the balloon through the jailed wire may occur in patients with a long proximal stented segment. Therefore, this technique is not recommended in this situation. The possibility of failure in the implantation of the SB stent theoretically exists; therefore, the technique, as well as the percutaneous approach of bifurcated left main, should be performed by expert operators.
Conflicts of interestNone declared.
Received 7 June 2010
Accepted 18 October 2010
Corresponding author: Servicio de Cardiología, Hospital Reina Sofía, Avda. Menéndez Pidal 1, 14004 Córdoba, Spain. manuelpan@telefonica.net