Permanent pacemaker implantation is a challenge in pediatric patients, who account for less than 1% of all patients who undergo this procedure.1 Widespread pacemaker use in children is limited by the absence of devices tailored to this population. Epicardial pacemaker placement used to be the preferred option for young patients, as the size of generators and endocardial leads were considered to be inappropriate and even dangerous for young children. Today, however, endocardial pacemakers are being increasingly used in the pediatric population as they offer several advantages, such as lower sensing and pacing thresholds and a reduced risk of lead fractures.2
We report our experience with permanent pacemaker implantation in patients weighing less than 10 kg at our hospital between January 2006 and March 2015. The procedure was performed in 25 patients with a median age of 17 months (range, 6-40 months) and a median weight of 7kg (range, 4.4-10.0 kg). The indication for pacing in 22 (88%) of the patients was complete atrioventricular block (AVB) after surgery (Table). The AVB had occurred after closure of a ventricular septal defect in all cases except one. The median time between surgery and implantation was 23 days (range, 9-40 days). The pacing leads were inserted by puncturing the right (n=15) or left (n=10) subclavian vein. The atrial or ventricular leads, measuring 52 cm in length and 2 mm in diameter, were inserted through 7-Fr introducer sheaths. Bipolar active-fixation leads were used in all cases. For dual chamber pacemakers, a right atrial loop measuring approximately 4 to 6 cm in length was created (Figure). Details of the generator models, implantation sites, pacing mode, and electrical parameters during implantation and follow-up are given in the Table. The generator was placed in a subpectoral pocket. The implantation procedure was completed without complications in most of the patients. One patient developed supraventricular tachycardia without repercussions during fixation of the atrial leads. In another patient, the atrial lead needed to be replaced due to malfunction caused by dislodgement on day 2 after the procedure. Twenty-two of the patients (88%) were followed up for a median of 48 months (range, 1-102 months). Generator replacement due to battery depletion was necessary in 2 children and there was no evidence of venous thrombosis during replacement in either case. Leads were extracted due to pocket-site infection in 3 patients (12%) at 8, 25, and 27 months. There had been no evidence of hematoma at the implantation site in any of the cases. Once the infection had cleared, the lead was removed using an epicardial surgical approach and replaced in 2 patients and was repositioned percutaneously on the contralateral side in the third. All the patients, including those who required lead extraction, were in good clinical health.
Clinical and Electrical Characteristics of Permanent Endocardial Pacemaker Implantation in Young Children
| Patient | Congenital heart disease | Generator | Implantation site | Pacing mode | Electrical parameters during implantation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrium | Ventricle | Atrium | Ventricle | Heart rate, max/min (bpm) | ||||||||
| Sensitivity (mV) | Pacing treshold (mV) | Impedance (Ω) | Sensitivity (mV) | Pacing threshold (mV) | Impedance (Ω) | |||||||
| 1 | ASD+ VSD | St JudeMicrony II | ------ | Apex | VVIR | ------- | ------- | ------- | N/A | N/A | N/A | 185/90 |
| 2 | CoAo + PDA + VSD | Guidant InsigniaI Entra | Posterior wall | Apex | DDDR | N/A | N/A | 464 | N/A | N/A | 512 | 150/80 |
| 3 | Double discordance + VSD | Vitatron | Posterior wall | Apex of LV | DDD | N/A | 0.2 | 450 | N/A | 0.4 | 598 | 170/90 |
| 4 | AVC defect | St. JudeMicrony II | ----- | RVOT | VVIR | ------- | ------- | ------ | N/A | N/A | 415 | 185/90 |
| 5 | VSD + PDA | Guidant InsigniaI Entra | Atrial appendage | RVOT | DDDR | N/A | N/A | 610 | N/A | N/A | 530 | 180/80 |
| 6 | Supracardiac-type TAPVC in SVC | Guidant InsigniaI Entra | Posterior wall | Apex | DDD | 3.5 | 0.4 | 420 | 3.5 | 0.4 | 430 | 180/90 |
| 7 | Dextrocardia, situs inversus, DORV | Guidant InsigniaI Entra | Posterior wall | Apex | DDD | 0.75 | 0.6 | 447 | 8.6 | 0.9 | 464 | 150/90 |
| 8 | Structurally healthy heart | Guidant InsigniaI Entra | Posterior wall | Apex | DDDR | 1.5 | 530 | 8.0 | 490 | 185/90 | ||
| 9 | TGA + VSD + subpulmonary ring | Guidant InsigniaI Entra | Lateral wall | Apex | DDDR | 2.0 | 1.0 | 400 | 10 | 1.25 | 700 | 185/110 |
| 10 | VSD + PDA | Medtronic | Atrial appendage | RVOT | DDDR | 0.19 | 2.4 | 866 | 15 | 2.1 | 952 | 180/80 |
| 11 | VSD + PDA | Boston ScientificAltrua | Atrial appendage | RVOT | DDD | 1.0 | 0.75 | 470 | 6.0 | 0.5 | 440 | 185/70 |
| 12 | Structurally healthy heart + paralysis of right atrium | Boston ScientificAltrua | Posterior wall | RVOT | DDDR | N/A | N/A | 420 | 8.5 | N/A | 480 | 185/90 |
| 13 | VSD + PDA | Boston ScientificAltrua | Atrial appendage | RVOT | DDDR | 2.0 | 1.6 | 610 | 15 | 1.2 | 530 | 180/70 |
| 14 | VSD + PDA | Boston ScientificAltrua | Posterior wall | RVOT | DDDR | 3.6 | 1.5 | 636 | 20.7 | 0.9 | 870 | 185/100 |
| 15 | Juxtaposed atrial appendages | Medtronic | Interatrial septum | Apex | DDD | 1.0 | 0.6 | 764 | 6.8 | 1.1 | 697 | 185/100 |
| 16 | VSD + PDA | Boston ScientificAltrua | Atrial appendage | RVOT | DDDR | 1.5 | 0.9 | 430 | 2.5 | 0.5 | 480 | 180/80 |
| 17 | AVC defect | Boston ScientificAltrua | Lateral wall | RVOT | DDDR | 1.2 | 0.5 | 780 | 7.0 | 0.8 | 640 | 185/100 |
| 18 | ASD + VSD | Boston ScientificAltrua | Posterior wall | RVOT | DDDR | 1.2 | 0.4 | 560 | 6.0 | 0.7 | 538 | 185/70 |
| 19 | VSD | Boston ScientificAltrua | Roof | Apex | DDDR | 0.75 | 0.8 | 413 | 2.5 | 0.4 | 445 | 180/80 |
| 20 | VSD + PDA | Boston ScientificAltrua | Lateral wall | Apex | DDDR | 0.75 | 1.2 | 534 | 2.5 | 1.2 | 568 | 180/80 |
| 21 | Intracardiac-type TAPVC in coronary sinus + VSD | Boston ScientificAltrua | Atrial appendage | RVOT | DDDR | 0.5 | 0.9 | 474 | 2.5 | 0.5 | 540 | 185/90 |
| 22 | DORV | Boston ScientificAltrua | Roof | RVOT | DDDR | 0.8 | 1.3 | 568 | 3.0 | 1.4 | 620 | 185/70 |
| 23 | CoAo + VSD | Boston ScientificIngenio MRI | Atrial appendage | RVOT | DDDR | 1.0 | 1.5 | 610 | 6.5 | 0.7 | 740 | 185/100 |
| 24 | VSD | Boston ScientificIngenio MRI | Atrial appendage | RVOT | DDDR | 0.75 | 0.6 | 468 | 2.5 | 0.8 | 534 | 185/90 |
| 25 | ASD + DORV | Boston ScientificIngenio MRI | Atrial appendage | RVOT | DDDR | 0.75 | 0.4 | 788 | 2.5 | 0.4 | 753 | 185/110 |
| Patient | Electrical parameters during follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atrium | Ventricle | Pacing % | AV interval | |||||||
| Sensitivity (mV) | Pacing threshold (mV) | Impedance (Ω) | Sensitivity (mV) | Pacing threshold (mV) | Impedance (Ω) | Atrial | Ventricular | SAV | PAV | |
| 1 | ----- | ----- | ----- | N/A | N/A | N/A | NF | NF | NF | NF |
| 2 | N/A | N/A | N/A | N/A | N/A | N/A | NF | NF | NF | NF |
| 3 | 1.75 | 2.5 | 650 | N/A | 0.75 | 450 | 29 | 99 | NA | NA |
| 4 | ----- | ----- | ----- | N/A | N/A | N/A | ----- | NA | ----- | ----- |
| 5 | 1.2 | 3.25 | 310 | 8.6 | 5.0 | 380 | 6 | 100 | NA | NA |
| 6 | N/A | N/A | N/A | N/A | N/A | N/A | NF | NF | NF | NF |
| 7 | 2.0 | 0.1 | 470 | N/A | 2.5 | 500 | 57 | 100 | NA | NA |
| 8 | 4.2 | 1.75 | 470 | N/A | 1.5 | 520 | 0 | 100 | 150 | 80 |
| 9 | N/A | N/A | N/A | N/A | N/A | N/A | 13 | 98 | NA | NA |
| 10 | 4.0 | 1.75 | 460 | 7.7 | 5.1 | 383 | 21 | 100 | 80 | 140 |
| 11 | 2.0 | 0.05 | 370 | N/A | 0.05 | 410 | 0 | 100 | NA | NA |
| 12 | 2.5 | 1.16 | 490 | 4.4 | 4.1 | 490 | 100 | 73 | NA | NA |
| 13 | 0.75 | 0.75 | 460 | N/A | 2.5 | 420 | 1 | 99 | NA | NA |
| 14 | N/A | 2.0 | 410 | N/A | 1.75 | 520 | 97 | 100 | NA | 150 |
| 15 | 1.4 | 1.25 | 522 | N/A | 1.25 | 470 | 1 | 100 | 120 | 150 |
| 16 | 2.4 | 1.24 | 610 | N/A | 1.25 | 480 | 4 | 100 | NA | NA |
| 17 | 2.2 | 1.75 | 420 | N/A | 1.5 | 420 | 19 | 100 | NA | NA |
| 18 | 0.5 | 1.5 | 420 | N/A | 1.5 | 420 | 14 | 100 | NA | NA |
| 19 | N/A | 2.0 | 380 | N/A | 1.75 | 410 | 2 | 100 | NA | NA |
| 20 | 1.0 | 1.25 | 530 | N/A | 1.75 | 430 | 40 | 100 | NA | NA |
| 21 | 0.32 | 8.75 | 560 | 6.5 | 1.5 | 440 | 14 | 92 | NA | NA |
| 22 | 2.4 | 1.25 | 410 | N/A | 1.25 | 460 | 57 | 100 | NA | NA |
| 23 | 1.9 | 1.25 | 400 | N/A | 1.5 | 460 | 11 | 100 | NA | NA |
| 24 | 0.9 | 6.25 | N/A | 12 | 2.0 | N/A | 7 | 2 | NA | NA |
| 25 | N/A | N/A | N/A | N/A | N/A | N/A | 56 | 100 | 80 | 160 |
ASD, atrial septal defect; AVC, atrioventricular canal; bpm, beats per minute; CoAo, coarctation of the aorta; DORV, doublet outlet right ventricle; LV, left ventricle; N/A, not applicable; NA, not available; NF, not followed up; PAV, paced AV; SAV, sensed AV; PDA, patent ductus arteriosus; RVOT, right ventricular outflow tract; SVC, superior vena cava; TAPVC, total anomalous pulmonary venous connection; TGA, transposition of the great arteries; VSD, ventricular septal defect.
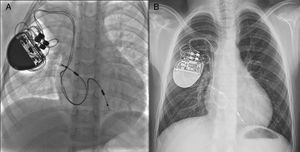
Two-year-old patient weighing 9kg with congenital atrioventricular block. Note the position of the leads in both the atrium and at the right ventricle apex (A). The same patient aged 8 years old and weighing 25kg (B). Note the minimum displacement of the leads despite the significant somatic growth.
A number of factors need to be considered when placing an endocardial pacemaker in young children. Lead displacement caused by growth can interfere with pacing, requiring the insertion of new leads within a relatively short period. Use of a longer atrial lead can prevent this from happening. Gheissari et al.3 calculated that a right loop of 8cm would allow a child to grow for 6 to 12 years without the need for reoperation and that approximately 10mm of lead per year should accommodate for somatic growth. Other authors, however, have warned that a surplus lead of this length could be displaced into the right ventricular outflow tract, possibly causing lung failure.4 In our experience, a loop length of approximately 4 to 6cm is adequate. We have detected no problems to date, although we acknowledge that larger series and longer follow-up times are necessary. Another important issue with pacemaker placement in young children is the thinness of the subcutaneous tissue layer, as the generator tends to exert tension against the tissue and can cause lesions. This increases the risk of infection and the need for extraction, as has been previously indicated.5 The use of subpectoral pockets has been associated with a lower risk of infection in such cases.6 Subpectoral placement is preferred not only for cosmetic reasons but also because of the greater protection provided by the pectoral muscle in young patients.
In conclusion, although patients require close lifetime follow-up due to the risk of venous thrombosis and the possible need for lead extraction, we consider that endocardial pacing in pediatric patients weighing less than 10kg is a reasonably safe and effective option in hospitals with experience.