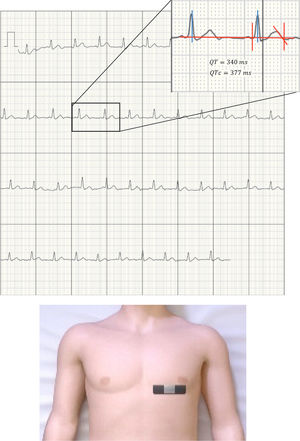
The pandemic caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is posing a major challenge to the international scientific community and to health care worldwide. The lack of effective treatments has obligated the experimental or compassionate use of drug combinations, so that most protocols include combinations of protease inhibitors (lopinavir/ritonavir), antimalarials (chloroquine/hydroxychloroquine), and antibiotics and immunomodulators such as azithromycin,1 among others. Many societies have already issued warnings about the use of these drugs and QT interval prolongation and the increased risk of sudden cardiac death from ventricular arrhythmias,2 further aggravated by the use of antiemetics and antidiarrheals for the relief of gastrointestinal symptoms. While effective therapeutic tools against the virus remain unavailable, efforts should be made to optimize the prescription and safety of currently used drugs. Given that these patients are in respiratory isolation, it is difficult to perform serial electrocardiograms (ECGs). Thus, the Food and Drug Administration has included among its recommendations the use of remote connection devices such as the KardiaMobile 6L (AliveCor, United States). This device has previously been approved for the detection of atrial fibrillation and QT monitoring in this setting3 and has already been mentioned in protocols such as that proposed by the Mayo Clinic.4 Although other devices with similar benefits are currently available, such as EKGraph (Sonohealth, United States), WIWE (myWIWE Diagnostics, Hungary), and Wecardio (BORSAM Biomedical Instruments, China), our hospital has chosen the AliveCor device for its use in the electrocardiographic monitoring protocol. The large volume of patients and the lack of experience with the aforementioned drugs have led to the acquisition of this device for monitoring the corrected QT interval (QTc). This approach has advantages over conventional ECG: ease of use, affordability, small size, remote data transmission (which minimizes the risk of contaminating the receiving device), and simplicity of disinfection in 70° alcohol. This device can obtain brief ECG recordings (30 s), allowing many patients to be monitored in little time. A receiver (mobile phone or tablet) is needed that connects via bluetooth with a range of at least 10 linear meters. Although the device provides 6 leads for the frontal plane of the ECG, for simplicity we decided to use the 1-lead option. There is another version of the device that only provides 1-lead ECG, but it is not equipped with a bluetooth connection and so it would need to be close to the receiver. Before starting the protocol, and as an internal validation process, QTc was measured in lead V5 on conventional 12-lead ECG and in the single lead of the Kardia device placed under the left breast or over the fingertips (figure 1). A series of 50 patients (33 patients with COVID-19 and 17 admitted to cardiology for other causes) underwent the protocol. An intraclass correlation coefficient of 0.902 was obtained (95% confidence interval, 0.811-0.950). No differences in QT measurement were observed between the fingertips and the thorax. Its placement on the chest was easier for nurses and the ECG recording in this area was subjectively better (higher voltage, allowing better differentiation of the T wave). Moreover, chest placement does not require the collaboration of the patient (figure 1).
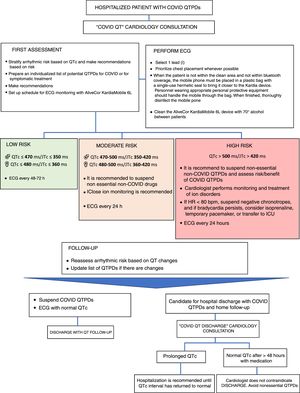
All patients with suspected or confirmed SARS-CoV-2 infection who needed hospitalization underwent baseline 12-lead ECG. The QT-interval monitoring protocol (figure 2) starts when the service managing the patient initiates treatments that carry a risk of QT prolongation. This service requests a cardiology consultation, immediately followed by assessment of the QTc interval (JTc in the case of wide QRS) using the AliveCor KardiaMobile 6L device for monitoring. Follow-up notes are entered in the patient's electronic health record, which includes arrhythmic risk stratification (see figure 2). Special attention is paid to concomitant drugs and ionic imbalances that may prolong the QT interval. Based on these findings, recommendations are made in conjunction with the treating physician and ECG monitoring with KardiaMobile is scheduled. To streamline the protocol during the current overload situation, we stratified arrhythmic risk using only the duration of the QT interval and chose a single-lead recording of 30 s, prioritizing placement in the chest whenever possible.
Since the start of the protocol, the QTc of 39 patients has been assessed (79.5% male; mean age, 62.4 ± 14.2 years). During follow-up, all patients received lopinavir/ritonavir, hydroxychloroquine, or azithromycin, in addition to medication for symptomatic relief. Prolonged QTc appeared in 6 patients (5 with QTc > 500ms and 1 with JTc > 420ms due to complete right bundle branch block), which was corrected when part of the medication was suspended as recommended by the cardiologist. Since the implementation of the monitoring protocol, no patient has died due to suspected ventricular arrhythmia associated with prolonged QT interval. Acceptance by the nursing staff has been very satisfactory, because it represents a simple alternative to the complexity of performing 12-lead ECG in these patients.