Keywords
INTRODUCTION
The thoracic esophagus is situated between the right and left pulmonary veins (PV) in direct contact with the left atrium (LA), and displays great interindividual anatomical variability.1 During circumferential pulmonary vein isolation (CPVI) in patients with atrial fibrillation (AF), radiofrequency lesions are produced in the LA and potentially lethal esophageal lesions can develop.2,3 This complication may be prevented by assessing the position of the esophagus during CPVI.4 We describe the use of a catheter designed for 3D reconstruction of the esophagus that enables analyzing its course and movements during the ablation procedure. We investigate alterations to the ablation strategy in areas close to the esophagus to reduce the risk of injury.
METHODS
The study included 20 consecutive patients undergoing CPVI for AF resistant to conventional treatment. Computerized tomography (CT) of the thorax was performed to assess the thickness and diameters of the esophagus in axial slices. Sedation was achieved with remifentanil and anticoagulation with sodium heparin. A circular mapping catheter for recording PV electrograms (Lasso, Biosense-Webster) and a 3.5-mm irrigated-tip ablation catheter (Navi-Star Thermocool, Biosense-Webster) were introduced into the LA via transeptal puncture. Reconstructions of the LA and PV were produced with independent maps using an electroanatomical mapping system (CARTO XP, Biosense-Webster) and the PV ostium identified according to changes in impedance and electric and fluoroscopic parameters.3
Mapping the Esophagus
Anatomical mapping of the esophagus was conducted using a dedicated catheter (Esophastar, Biosense-Webster) introduced via a naso-esophageal approach and withdrawn postero-cranially, thereby constructing a point-by-point 3D map and a virtual esophageal tube. The dimensions of the map and virtual tube, procedural time and concordance were compared to the CT measurements in each reconstruction.
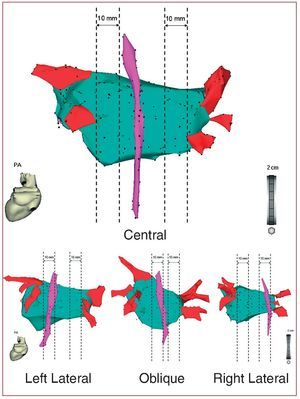
To determine the position of the esophagus in relation to the LA, we selected the view offering the greatest elongation of the posterior LA starting from a posteroanterior projection of the 3D map. From the midmost portion of the right and left PV ostia, we measured the distance to the homolateral border of the esophagus. The courses were classified (Figure 1) as right or left lateral if they were located at least 10 mm from the PV and central otherwise. If they were less than 10 mm from the PV and crossed the center of the area of interest—defined as the point equidistant to the contralateral PV—the course was considered oblique. The esophageal catheter remained in place until the end of the procedure and reconstruction performed at the beginning, during and at the conclusion of the procedure. Movement of the esophagus was suspected if the catheter tip crossed the boundary of the initial map, and was considered significant if >10 mm. Patient tolerance to the catheter was measured on a 1-5 scale (1 = no discomfort; 5 = unbearable).
Figure 1.Point-to-point esophageal map and classification of the course of the esophagus.
Ablation
All patients underwent CPVI by homolaterally encircling the PV at more than 5 mm from the ostium (margin of ablation), until the atrial electrogram voltage decreased by >90% or to <0.05 mV, delivering radiofrequency energy applications of 35 W at a maximum temperature of 45oC. We altered the ablation lines individually to locate them more than 5 mm from the border of the virtual esophagus (safety margin). In the lateral or oblique courses, due to inability to stay within these margins, we decreased the energy application to 25 W and the pulses to less than 20 s. When we could not obtain complete PV isolation (the aim of the ablation procedure) via encirclement, we applied it within the circlet.
The SPSS 14 statistical package was used in all cases. ANOVA or the Student t test were used to identify statistically significant differences between means, and c2 or Fisher's exact test for qualitative variables.
RESULTS
Table 1 shows the population characteristics. It was possible to introduce the catheter in all the patients with a good level of tolerance—median, 2 [p25-75, 2-3]—and without complications. The position of the esophagus varied, with 13 central courses (65%; minimum distance to PV, 17 [3] mm), 6 lateral— 4 right (20%; distance, 6.7 [2.3] mm) and 2 left (10%; distances 7 mm and 5 mm)—and 1 oblique (5%; minimum distance, 6 mm) (Figure 1). There was no association between the course of the esophagus and the anatomical, clinical or demographic variables analyzed. In the CT, the esophagus presented elliptical morphology in axial slices; the lateral diameter was greater than the anteroposterior (18.7 [3] mm and 10.1 [1.5] mm) and mean wall thickness was 3.8 (0.3) mm. The diameter obtained with the point-to-point map was 10 (3) mm and with the tube, a fixed value (10 mm). The addition of 5 mm on each side of the virtual esophagus as safety margin was greater than the lateral diameter of the esophagus in CT in 95% of the maps and 80% of the tubes. The duration of esophageal mapping was 2.9 (0.5) min (41 [8] points). Only a few seconds were required for the tubes.
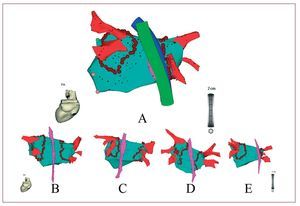
There were no significant movements (Figure 2A). We altered the ablation strategy in 10 patients. In 3 (15%) central courses within 10 mm of the ostia, the radiofrequency energy was applied closer to the ablation margins (1 left and 2 right) while respecting the safety margin. In 7 (35%), we reduced the radiofrequency energy and application time; this involved 6 lateral courses (2 left and 4 right) in the entire posterior part of the homolateral circlet and in 1 oblique in the posterosuperior part only (Figure 2B). During a follow-up of 4 (2) months, there were no complications and 70% of the patients remained free from arrhythmias.
Figure 2.A: initial esophageal reconstruction (pink), during the procedure (green) and at the end (blue), without significant movement. B-E: the same cases as shown in Figure 1 indicating the ablation lines according to the position of the esophagus. In C, D, and E it was necessary to reduce the radiofrequency energy and application time.
DISCUSSION
Esophageal 3D cartography using a dedicated catheter provides the exact location of the esophagus during CPVI in a simple and well-tolerated way, which aids in confirming its stability during the procedure. Altering the ablation strategy to reduce potential risk without changing the aims of ablation is very common.
Damage to the esophagus has been recently described as a complication of LA ablation.2,3 This is due to the thermal lesion caused by radiofrequency energy applications in atrial regions in direct contact with the esophagus, and can range from minor local lesions to atrio-esophageal fistulas.5 Although the incidence of fistulas is rare, they are a difficult complication to diagnose and have high mortality, and thus avoiding the LA regions adjacent to the esophagus has become a widely used approach for minimizing the risks involved in ablation.3
Different imaging techniques have been used to locate the esophagus, but all of these involve limitations.3 The incorporation of esophageal anatomy into 3D maps provides accessible and real information. Previous studies have validated this technique using different navigation systems,4,6 using more rigid catheters, and that normally require the need for general anesthesia. However, the use of a dedicated catheter is simple and well-tolerated and can be performed with mild sedation.
The position of the observed courses was independent of all the variables analyzed. Lateral movements during ablation have been described, but have been overestimated in studies using oral contrast media, since the need to swallow and the persistence of the contrast agent facilitate peristalsis.7 Like other authors,8 we did not observe significant movements when mild sedation was used, suggesting that a single initial map would be sufficient to locate the danger zones.
Point-to-point maps are more accurate than standardized tubes and do not substantially lengthen the time of procedure. This approach maps the esophageal bed; thus, a 5-mm increase in the margin at each side of the map more accurately recreates the dimensions of the esophagus as measured by CT. If tubes are used, with a fixed diameter of 10 mm, these margins should be >5 mm in up to 20% of the cases.
If it is not possible to stay within the safety and ablation margins, then a closer approach to the esophagus or PV is required, which increases potential risk.3 In courses around 10 mm away from the ostia, a small alteration in the ablation strategy was considered sufficient, but in lateral or oblique courses greater changes were made by reducing radiofrequency energy and application times.
Limitations
Although apparently useful, it cannot be confirmed if visualization of the esophagus would have a real clinical impact in reducing atrio-esophageal fistulas, given their low incidence. Long-term follow-up will determine the possible influence of modifications on ablation outcomes.
In conclusion, 3D monitoring of the esophagus demonstrates its great anatomical variability and confirmed its stability during CPVI using mild sedation. Point-to-point esophageal maps are more accurate than tubes standardized for movement. Guiding the radiofrequency energy applications according to the esophageal anatomy involves altering the ablation strategy in a large number of cases.
Adriano Jiménez Velasco is an engineer and clinical assistant at Biosense Webster.
Correspondence: Dr. E. Arana-Rueda.
Avda. España, 67, 1.o C, 41700 Dos Hermanas. Sevilla. España.
E-mail: eduaru@ono.com
Received July 23, 2008.
Accepted for publication February 3, 2009.