Keywords
The phenomenon of adverse ventricular remodeling after an ST-elevation acute myocardial infarction (STEMI) is characterized by early dilatation in the necrotic and peri-infaction region, with subsequent spread to initially healthy areas of the myocardium. This leads to changes in ventricular shapes and sizes (sphericity and dilatation) leading in turn to systolic dysfunction and heart failure.1 This phenomenon affects almost a third of the survivors of an infarction and is associated with poor prognosis.2
The most effective measures for preventing ventricular remodeling include the early administration of reperfusion treatments (thrombolysis and primary angioplasty) and pharmacological neurohormonal blockade.3 However, the early revascularization of the culprit artery in the epicardium is not always associated with successful microvascular reperfusion,4,5 and so a considerable percentage of patients suffer the so-called "no reflow" phenomenon, which is predictive of complications and ventricular dilatation in the short and long term.6 From the histological point of view, the "noreflow" phenomenon has been characterized in detail and has been related to inflammatory and thrombotic phenomena that cause obstruction in the microvascular bed.7-9
Attempts have been made to assess the success of microvascular reperfusion after an acute coronary event by means of different techniques such as coronary angiography,10,11 scintigraphy,12 positron emission tomography,13 coronary Doppler techniques,14 contrast myocardial perfusion echocardiography,4 and contrast-enhanced magnetic resonance imaging.5 Two of the main advantages of late contrast-enhanced magnetic resonance imaging (cMRI) compared to most imaging techniques are its noninvasive nature and its high spatial resolution, which, apart from detecting microvascular obstruction and determining myocardial viability, can provide detailed information on the morphological and functional sequelae after acute myocardial infarction.15
Microvascular obstruction has been described, in the context of STEMI in cMRI, as areas of no signal within an area of enhanced signal in the subendocardium.16 This can been seen in sequence images during the first pass of the contrast-in the first few minutes after gadolinium injection-as well as later (between 5 and 15 minutes) in contrast-enhanced images,17 and has been shown to have prognostic implications for predicting subsequent ventricular dilatation.5,15
Our aim was to analyze the impact of persistent microvascular obstruction (PMO) detected by cMRI15 on the course of postinfarction ventricular remodeling in a small cohort of successfully revascularized patients who were submitted to a new anti-remodeling therapy in clinical development—intracoronary implantation of mononuclear autologous bone marrow mononuclear cells (ABMMC).
METHODS
The study protocol was drafted in accordance with the Declaration of Helsinki and was approved by the local ethics committee. All patients signed an informed consent after receiving a detailed explanation of the protocol.
Patients
The study included 14 patients aged over 18 years who were admitted to our center with diagnosis of extensive STEMI at any site (sum of elevated ST >6 mm), who successfully underwent reperfusion treatment within 72 hours of the onset of symptoms (grade 3 TIMI epicardial flow after primary angioplasty or post-thrombolytic angioplasty with tenecteplase) and with an identifiable area of asynergy in the ventriculography.
Patients were excluded if they were in cardiogenic shock, had a mechanical complication of the infarction, were contraindicated for MRI studies (implantable defibrillator-cardioverters or pacemakers), had a history of cancer in the previous 5 years, or had any disease that might compromise survival during the study period.
Extraction, Processing, and Cell Implantation
Fifty milliliters of bone marrow were taken from each patient by means of repeated puncture of the posterior iliac crest with a punch (5 mL per puncture) while under mild sedation and analgesia, with monitoring of blood pressure, electrocardiogram, and oxygen saturation. The sample was introduced into a bag for transport along with 20 mL of heparinized peripheral blood of the patient. A 5 mL sample was sent to the cryopreservation laboratory for cytometry and culture.
The mononuclear fraction was obtained by the gradient density method in Ficoll before lysing the erythrocytes with water. The cell suspension was resuspended in RPMI-1640 medium with 2% of autologous plasma. The number of cells was adjusted to 1´106/mL. The mononuclear cells were transferred to a Teflon bag and incubated overnight at 37ºC with 5% carbon dioxide. The following day, the cells were centrifuged and heparinized, and their viability was tested with trypan blue.
Cell implantation in the infarction region was done in the first 2 weeks after the infarction in the catheterization laboratory with the patient still conscious and with monitoring of blood pressure and the electrocardiogram. The suspension of ABMMCs was introduced into a 50 mL syringe connected to the infusion catheter and the cell preparation was infused into the coronary artery through an angioplasty balloon catheter inflated to low pressure (2-4 atm) in the previously stented lesion, beforehand to prevent reflux of the solution and to increase the contact time with the coronary bed. To prevent additional ischemic damage, during transfer, periods of perfusion at 1 mL/min for 2 minutes (occlusion of the coronary artery) were alternated with 1-minute periods of reperfusion (deflation of the balloon).
Cardiac Magnetic Resonance Imaging Studies
The protocol included carrying out cardiac magnetic resonance imaging studies in the first 2 weeks and at 10 months after infarction.
All studies were carried out with an MRI device (Signa, General Electric) at a field strength of 1.5 T with a specific phased-array antenna for cardiac studies. The left ventricular (LV) functional status was determined before administration of contrast with an echo-gradient sequence (FIESTA) with electrocardiogram-gating and multiple in-breaths (repetition time/echo time 3.2/1.6; orientation angle, 60º; matrix size 256´128 mm; visual field, 320 mm). Parallel fast-capture image sequences were not available during the study. Multiple basal-to-apical views of the LV short axis were obtained with a slice thickness of 8 mm and separation between planes of 1 mm. In addition, 2, 3, and 4 chamber views were obtained. The late contrast-enhanced images were taken 10 minutes after manual injection of gadolinium 0.2 mmol/kg (gadolinium-DTPA) using a sequence with inverse pulse recovery and echo gradient. The inversion time was adjusted to cancel out the myocardial signal. The size of a typical matrix was 195´192 mm and the visual field was 320 mm. The views obtained with the late-enhancement sequence were the same as those selected for the LV functional status.
Analysis of Cardiac Magnetic Resonance Imaging
A quantitative analysis was undertaken of the ejection fraction, end-systolic volume, end-diastolic volume, and mass and thickness of the LV wall by manual planimetry of the end-diastolic and end-systolic endocardial and epicardial borders (excluding papillary muscles) in each of the short planes acquired in the cine-MRI images.
In these late contrast-enhanced images, the size of the infarction was quantified by manual planimetry of the enhanced contrast uptake in short LV axes views. We also analyzed the presence of PMO, defined as a zone of low contrast uptake in the middle of an infarction zone (Figure 1).
Figure 1. Four-chamber slice (A) and apical axis slice (B) of the baseline cardiac magnetic resonance imaging study of the same patient. The black arrow indicates an area of persistent microvascular obstruction (hypointense signal localized in the middle of an infarcted region with signal enhancement).
Both types of analyses were undertaken with MASS 4.0.1 software in the Cardiac Imaging Unit of ICICOR by an experienced observer (P.T.) who was unaware of the clinical course of the patients or of the findings of the other examinations included in the protocol.
Statistical Analysis
The categorical variables were expressed as absolute value and percentage and the continuous ones as mean (SD) and/or median and interquartile range. The normality of the quantitative variables was tested by the Shapiro-Wilk test. A posteriori, 2 study groups were established according to whether or not the patient had PMO in the baseline cMRI study. The comparison between groups of categorical variables was done by means of the c2 test and the exact Fisher test when necessary. For continuous variables, the Student t test or its nonparametric equivalent, the Mann-Whitney U test, was used if the data were not normally distributed. For the evaluation of changes from baseline during follow-up of the continuous variables in all the patients, the Student t test was used for paired data, and in the case of non-normally distributed data, the nonparametric equivalent, the Wilcoxon test, was used.
Data were analyzed with the SPSS software package, version 14.0 (Chicago, Il, USA). Statistical significance was established at P<.05.
RESULTS
The 14 patients included (age, 59 [12] years, all men) received intracoronary infusion of 66 (39)´106 ABMMCs, 8 (2) days after the onset of symptoms. The patients were divided into 2 groups according to the baseline cMRI study: those with PMO (5 patients) and those without PMO (9 patients).
No significant differences were found between the groups in terms of demographic variables, infarction site, maximum creatinine kinase level, reperfusion treatment received, use of glycoprotein IIb/IIIa inhibitors, pharmacological treatment on discharge, and variables related to the cell transplant procedure such as duration, number of ABMMCs implanted, and phenotype of the implanted cells (Table 1). In the analysis of the immediate impact of the infarction according to baseline cMRI, a tendency was detected in the groups of patients with PMO to present with a larger infarction size, larger ventricular volumes, and worse LV general and regional systolic function (Table 2).
Cardiac Magnetic Resonance Imaging Studies
Baseline cardiac MRI studies were done 8 (4) days after infarction, and follow-up was at 313 (50) days. In the overall patient group, the LV ejection fraction showed a tendency towards improvement during follow-up (baseline LVEF, 46% [8%] compared to follow-up, 51% [11%]; P=.10). Likewise, there was a significant reduction in the infarction size assessed by late enhancement both in absolute mass (baseline, 42 [19] g vs follow-up, 26 [15] g; P=.001) and relative mass with respect to the total LV mass (baseline, 32% [11%] vs follow-up, 23% [11%]; P=.001). The characteristic PMO image was not observed in any of the follow-up cMRI studies.
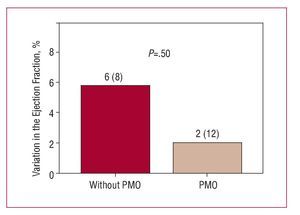
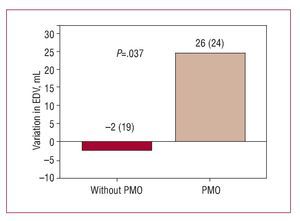
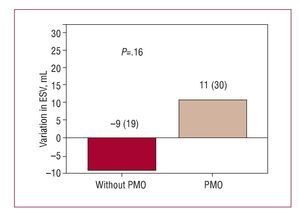
In the comparative group analysis, no significant difference was found between patients with and without PMO in the increase in LVEF (2% [12%] vs 6% [8%]; P=.5) (Figure 2) or in the reduction in infarction size during follow-up (-16 [4] g vs -16 [9] g; P=.97). However, during follow-up, having PMO was associated with adverse ventricular remodeling, characterized by an unfavorable and opposite change in ventricular volumescompared to those without PMO; the variation in LV end-diastolic volume was significant (P<.037) (Figure 3) but not the end-systolic volume (Figure 4).
Figure 2. Variation of the left ventricular ejection fraction in follow-up according to the presence or not of persistent microvascular obstruction (PMO) in the baseline cardiac magnetic resonance imaging.
Figure 3. Variation in end-diastolic ventricular volume (EDV) at follow-up according to presence or not of persistent microvascular obstruction (PMO) in the baseline cardiac magnetic resonance imaging.
Figure 4. Variation in end-systolic ventricular volume (ESV) at follow-up according to presence or not of persistent microvascular obstruction (PMO) in the baseline cardiac magnetic resonance imaging.
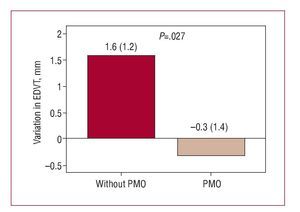
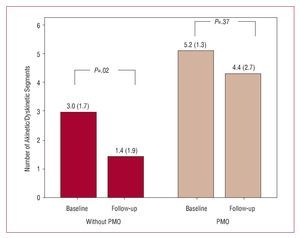
In turn, an increase in end-diastolic thickness was detected in patients with PMO (Figure 5) in clear contrast with the changes in the patients without PMO (P<.027). Moreover, only patients without PMO showed a significant reduction in the number of akinetic or dyskinetic segments compared to the baseline study (Figure 6).
Figure 5. Variation in end-diastolic ventricular thickness (EDVT) at follow-up according to presence or not of persistent microvascular obstruction (PMO) in the baseline cardiac magnetic resonance
Figure 6. Variation in number of akinetic or dyskinetic segments in the baseline regional contractility analysis and at follow-up according to presence or not of persistent microvascular obstruction (PMO) in the baseline cardiac magnetic resonance imaging.
DISCUSSION
In our study, we attempted to analyze the impact of PMO on the change in postinfarction ventricular remodeling in a small cohort of patients with STEMI who had been successfully revascularized and who underwent intracoronary implantation of ABMMCs. To evaluate the presence of PMO, we used late gadolinium-enhanced images in the cardiac MRI study. This technique, in addition to allowing a complete morphological and functional analysis of the left ventricle, shows PMO in the subendocardial area of the infarcted area (Figure 1) much more objectively and in much more detail than other noninvasive imaging techniques such as scintigraphy17 (with limited spatial resolution) or myocardial contrast echocardiography18 (whose use is restricted in the context of recent acute coronary syndrome). Unlike myocardial contrast echocardiography, cMRI can be used in this clinical context, except in patients with severe renal function impairment.
Our observations indicate that, in patients undergoing intracoronary implantation of ABMMCs, PMO is also related to adverse ventricular remodeling in the medium term. This process represents an unresolved obstacle in the current methodology for applying this new investigational therapy. Likewise, this study highlights the extraordinary capacity of this imaging technique to assess the individual risk of suffering ventricular dilatation, and as a result, heart failure, after acute myocardial infarction.
So far, the clinical trials that have assessed the safety, feasibility, and efficacy of intracoronary transplantation of ABMMCs after STEMI offer generally promising results,19 indicating that this therapy is safe and might provide a modest benefit in terms of improved systolic function, reduced infarction size, and reduced left ventricular volume, in addition to that provided by revascularization and pharmacological therapy. However, it is of note, particularly in the case of ABMMC transplantation, that the baseline ejection fraction of patients assigned to cell therapy in the randomized controlled studies published up until now is, at worst, slightly reduced (mean baseline LVEF, 48%-55%).20-24 Interestingly, among the results reported, we find that a considerably greater benefit was observed in the subgroup of treated patients with the worst baseline ejection fraction in the REPAIR-AMI study.24 Likewise, in the study conducted by Janssens et al,22 an increase was detected in the metabolic activity of the infarcted area only in large infarctions, as well as an improvement in the regional contractility in patients with transmural scarring who received cell infusions, even though no benefit was observed in overall ventricular function. Bearing in mind that, in both studies, patients in a worse functional or morphological state benefitted most from intracoronary cell implantation, some authors have suggested that, for future clinical trials, the inclusion criteria should be restricted to populations at high risk of developing ventricular remodeling.25,26
However, although microvascular obstruction has been shown to be an independent prognostic indicator of postinfarction morbidity and mortality and, therefore, a risk marker in these patients, to date, only 2 randomized clinical trials have assessed the impact of such obstructions on the effect of intracoronary implantation of bone marrow stem cells, with contradictory results. In the case of the BOOST trial, the presence of microvascular obstruction was shown not to be a predictor of recovery of overall systolic function in the long term (18 months postinfarction).27 In contrast, according to Janssens et al,22 this complication is associated with a lack of functional recovery and predicts ventricular dilatation, regardless of the treatment to which patients were assigned (cell therapy or conventional treatment alone).
Our analysis supports the findings of Janssens et al,22 and points to the need to optimize the current methodology of stem cell therapy in acute postinfarction to achieve positive results in patients with PMO. Given that myocardial nesting of stem cells is generally poor and that microvascular obstruction of the occluded or destroyed capillary bed would prevent, a priori, stem cells implanted intravascularly from reaching their target, new therapeutic combinations (specific cell phenotypes, alternative administration routes, enhanced paracrine effect with cytokines) should be the object of future studies in the context of STEMI.28,29Limitations of the Study
The present study has 2 obvious limitations. First, we do not have a randomized control group to assess the effect of PMO in patients treated conventionally. Our patients were selected consecutively from a larger cohort (72 patients) that formed the population sample of a pilot phase I study, designed to assess the feasibility and safety of intracoronary implantation of ABMMCs after reperfused STEMI. The initial results of this study have already been published in this journal.30 For this reason, our observations are limited to indicating the association of PMO with adverse remodeling in a group of patients submitted to cell therapy, and in no case should conclusions be drawn on the benefit of this technique for patients participating in the study compared to standard postinfarction treatment. Second, an important limitation is the small sample size of patients, which had it been larger, would probably have revealed significant differences in terms of baseline morphological and functional abnormalities of the myocardium between groups and in terms of recovery of overall ventricular function or the change in end-systolic volume. In turn, a larger sample size would have allowed an analysis of the impact of quantitatively evaluated PMO (ventricular volume and absolute and relative mass with PMO). Nevertheless, the low variability in the cardiac MRI studies allows conclusive results to be obtained with sample sizes up to 80%-90% smaller than if we use other more widespread imaging techniques such as echocardiography.31 Likewise, we know that the presence of PMO is related to greater infarction size and worse immediate and medium- to long-term postinfarction ventricular function. What the finding of significant ventricular dilatation in patients with PMO with such a limited sample size does reveal is the enormous usefulness of this variable for predicting adverse ventricular remodeling on its own, as well as the usefulness of cardiac MRI for exactly detecting its presence.
CONCLUSIONS
Persistent microvascular obstruction, evaluated soon after an infarction by cardiac MRI with late contrast enhancement is associated with adverse ventricular remodeling in patients who undergo intracoronary implantation of ABMMCs following reperfusion. As a result, it seems necessary to optimize the current methodology of cell implantation to achieve positive results in these patients.
ABBREVIATIONSABMMC: autologous bone marrow mononuclear cells
cMRI: contrast-enhanced magnetic resonance imaging
LV: left ventricular
PMO: persistent microvascular obstruction STEMI: ST-elevation acute myocardial infarction
This study was made possible in part by support from the Cardiovascular Disease Network (REDCAVA) and the Network Center for Regenerative Medicine and Cell Therapy of Castille-Léon, Spain. The financial sources did not participate in any way in the study design, data collection, analysis, and interpretation of data, drafting of the manuscript, or the decision to submit the manuscript for publication, and they did not have any other relationship with the author that might be considered a conflict of interest.
Correspondence:
Dr. A. Villa.
Instituto de Ciencias del Corazón. Hospital Clínico Universitario. Avda. Ramón y Cajal, 3. 47005 Valladolid. España.
E-mail: adolfovilla74@hotmail.com
Manuscript received July 18, 2007.
Accepted for publication March 4, 2008.