Keywords
INTRODUCTION
One main application of nuclear cardiology is to assess the prognosis of ischemic heart disease patients. Myocardial perfusion studies using SPECT imaging provide a better prognostic information than clinical, stress test and angiographic data for predicting death or non-fatal infarctions.1-3 It has also been confirmed that isotopic ventriculography EF, at rest and exercise, can predict the incidence of hard cardiac event in ischemic heart disease patients.4-7 Given that myocardial perfusion and ventricular function offer a valuable prognostic information, both variables have been analyzed together to find out if they provided more information than separately, and different conclusions have been reached.8-12
Large population, randomized ischemic heart disease studies have demonstrated that surgical coronary revascularization improves long-term survival in high-risk patients with extense coronary disease or left ventricular dysfunction.13,14 Percutaneous revascularization has proven as effective as surgical revascularization reducing to mortality in high-risk ischemic patients.15 Nevertheless, there are very few studies performed with the aim of analyzing the mid-long term prognostic factors in revascularized patients.16,17 Prognosis evaluation of patients with coronary revascularization is certainly of interest, as it provides information about cases needing major supervision and therapeutic resources.
Gated-SPECT studies allow simultaneous analysis of myocardial perfusion and left ventricular function,18,19 and will prove to be an effective technique for assessing the prognosis of ischemic heart disease patients. Studies aimed at evaluating the prognostic capacity of gated-SPECT20,21 have been very few until now, and none has been performed in revascularized patients. The purpose of our study was to analyze, in a revascularized ischemic heart disease population, if variables of a gated-SPECT study performed before revascularization would predict mid and long term prognosis, by comparison with other clinical or angiographic variables.
METHODS
Population
Studied population consisted of 110 patients with ischemic heart disease who underwent percutaneous or surgical coronary revascularization between April, 1997, and September, 2000, in the Hospital do Meixoeiro-Instituto Gallego de Medicina Técnica in Vigo. Those patients for which a gated-SPECT study was not feasible or with a result of possible artifacts (absence of stable R-R due to atrial fibrillation, intraventricular conduction block, pacemaker rhythm, etc.), were not included. All patients were followed-up for at least one year (mean period, 23.7 months and longest follow-up period, 44 months). Patient´s conditions were evaluated by the same person by a telephone interview. All patients were contacted. Occurrence of death events and also combined clinical events (death, non-fatal infarction or hospital re-admission for cardiac reasons) was inquired.
Clinical data (Table 1)
The population included mostly male patients (87.3%), with a majority presenting a history of myocardial necrosis (78.2%). Most patients were revascularized due to acute ischemic event (recent infarction -- less than one month--, in 57.3%, and unstable angina, in 15.5% of cases). In 9 patients (8.7%) revascularization was motivated by heart failure. One third37 also referred comorbidity (chronic illness added to myocardial ischemia, such as respiratory failure, renal failure, peripheral vascular diseases and others).
Catheterization and coronary revascularization
Catheterization consisted in a coronary angiography and contrast ventriculography in 30° RAO projection. Coronary lesions were quantitatively analyzed and considered significant if obstruction percentage was 50% higher than vessel reference diameter. LV ejection fraction was calculated using contrast ventriculography (Dodge method).
All patients included were indicated for coronary revascularization, mostly percutaneous (100 cases, 90.9%). The remaining 10 patients underwent surgical revascularization. All the revascularization procedures were successful, defined as absence of death or periprocedural infarction (new Q-waves appearing in electrocardiogram). Seventy-one of the 100 percutaneously revascularized patients underwent an angiographic revision before 6 months, guided by protocol at first and afterwards and symptom-related latter on. In percutaneously revascularized patients, clinical restenosis was defined as symptoms reappearing within 6 months after procedure, with angiographic confirmation of a higher than 50% obstructive lesion in the treated area.
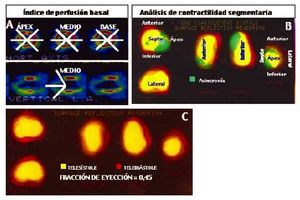
Sestamibi gated-SPECT study (Figure 1)
Fig. 1. Variables determined by gated-SPECT at rest. A. Baseline perfusion rate, calculated by using sestamibi captation analysis in 20 segments located at the apical, midline and baseline coronal axis levels and at midline vertical slice. High scores indicate increased severity and extension of captation defect. B. Regional wall motion analysis, by dividing the left ventricle 3D profile in 5 regions. The asynchronous regions were considered as those showing a major extension codified in green. C. End-systolic (in yellow code) and end-diastolic (in red code) ventricular profiles, with calculated left ventricular ejection fraction.
After catheterization and before revascularization, patients underwent a gated-SPECT study after receiving at rest 30 mCi of technetium-99m sestamibi. A dual head Sopha DST® scintigraphy camera with semicircular travel was used. Image acquisition was synchronized with the electrocardiogram R-wave, obtaining 8 images per cardiac cycle from end-systole to end-diastole. The commercially available MultiDim® Gated SPECT system was used for automatic processing. Perfusion tomographic slices for three standard axes (short or coronal, long vertical and long horizontal), and a left ventricular cavity 3D representation, were generated in each study.
Myocardial perfusion was evaluated with semiquantitative analysis of end-diastolic tomographic slices to create a baseline perfusion rate. A model already described20 was used, consisting basically in selection of three short or coronal axis slices (apical, midline and baseline), and the long vertical axis midline slice. Each short axis slice was divided in 6 segments, whereas the long vertical axis slice was divided in two apical segments, for a total of 20 segments. Each segment has a score depending on its captation activity: 0, normal (red coded); 1, erroneous or dubious captation; 2, moderate hypocaptation (yellow coded); 3, important hypocaptation (green), and 4, severe hypocaptation or null captation (blue or black).
The left ventricle 3D representation was created by an automatic system based on bead distribution along the left ventricle wall: two ellipsoid figures are generated at end-systole and end-diastole by radii directed from the ventricular cavity center to the wall portion with higher bead activity. End-systolic and end-diastolic volumes were calculated in this way, together with left ventricular EF.21
The ventricular 3D representation also permits regional wall motion analysis, after its division in five myocardial regions (apex, anterior, septum, inferior and lateral); the software codifies in green the asynchronous ventricular silhouette regions. The number of synchronous regions at rest was measured to identify acynetic or discynetic myocardium extension.
Statistical analysis
Statistical analysis was computed using SPSS/PC for Windows. As this is a cohort prospective study, continuous variables such as age, left ventricular EF by contrast ventriculography and gated-SPECT, and baseline perfusion rate, were transformed to categorical variables. The association degree of the studied variables (gender, age, smoking, hypercholesterolemia, diabetes, arterial hypertension, history of necrosis, comorbidity, reason for catheterization, number of injured vessels, complete revascularization, contrast ventriculography EF, baseline perfusion rate, number of asynchronous regions in gated-SPECT, gated-SPECT ejection fraction) and death or combined clinical events was analyzed by means of contingency tables (χ²). For follow-up analysis of patients, the rate of events is as important as the moment in which they occur, so survival was analyzed also. Kaplan-Meier survival curves were generated for discreet values of each variable, comparing the mean time free of events in both patient groups. Cox regression analysis was used for multivariate analysis, justified by events appearing in different moments of follow-up. Variable correlation was also analyzed with the Pearson test for parametric variables and the Spearmanρ test for non-parametric variables.
RESULTS
Angiographic, revascularization procedure and gated-SPECT characteristics of total population (Table 2)
Coronary disease extension was mainly one vessel (73.6%), and three vessels in 5.5% of cases. The left anterior descending artery was most frequently affected (61.8%). Mean left ventricular function determined by catheterization was impaired (0.46±0.11), and a lower than 0.50 ejection fraction was found in 57.3% of patients. Revascularization was performed mainly by stent implantation (77.3%), and was complete in 83.6% of cases.
The gated-SPECT studies showed important alterations in both global and ventricular regional wall motion analysis. One third of patients (36 cases, 32.7%) had an EF¾0.30 calculated with this method. Only 15 patients (13.6%) did not present a 3D ventricular representation showing a discynetic segment and 47 cases (42.8%) presented >=2 asynchronous regions. Ejection fraction calculated by gated-SPECT correlated significantly with values obtained by angiography (r=0.55; P<.001). Among the gated-SPECT variables, EF was significantly and inversely associated with the number of 3D ventricular representation asynchronous regions (r=-0.59; P<.001).
Events during follow-up
During a maximum of 44 months follow-up period, of the 110 included patients, 14 died and 36 suffered a combined clinical event (total events were 14 deaths, 6 non-fatal infarctions and 31 hospital re-admissions). Thus, the annual rate of events was 16.5% and 6.4% for death events.
As for percutaneously revascularized patients, 15 presented clinical restenosis and all these patients suffered at least one event: 5 deaths, 3 non-fatal infarctions and 13 hospital re-admissions for cardiac reasons. Of the 85 percutaneously revascularized patients not presenting clinical restenosis, a combined event was registered in 16 cases: 6 deaths, 3 non-fatal infarctions and 14 hospital re-admissions due to cardiac reasons. A second revascularization of the treated vessel was performed in 13% of population.
Variables related to mortality (Table 3)
In the total population (n=110), we analyzed clinical, angiographic and gated-SPECT study parameters that could be related to mortality. By univariate analysis, clinical variables significantly to death events were age over 60 years (P=.02), arterial hypertension (P=.01) and comorbidity (P=.04). As for the gated-SPECT variables, number of asynchronous regions (P=.007) and the gated-SPECT baseline EF<=0.30 (P=.007) were significantly associated to mortality. None of the angiographic study variables was associated to mortality.
Cox regression analysis for predicting mortality
During follow-up, baseline EF by gated-SPECT was independently related to mortality; as a result, severe deterioration of the gated-SPECT ejection fraction was found to increase five-fold the death event risk (OR=4.8; 95% CI, 1.6-14.6).
Survival curves without death events as per gated-SPECT ejection fraction (Figure 2A)
Fig. 2. Kaplan-Meier survival curves without death events in presence or absence of severe ventricular dysfunction determined by gated-SPECT. A. Total population analysis. B. Analysis of the population without clinical restenosis analysis.
After revascularization, patients with gated-SPECT baseline EF<=0.30 presented a significantly shorter mean survival than all other patients: 33 months (95% CI, 28-38), compared with 42 months (95% CI, 40-44 months); P=.002.
Variables associated to combined clinical events (Table 4)
Univariate analysis of clinical characteristics evidenced that a history of diabetes (P=.004), <60 years of age (P=.02) and comorbidity (P=.03) were associated to the incidence of combined events. Absence of complete revascularization was associated significantly with occurrence of an event (P=.005). An EF<=0.40 was also related with event rate (P=.02). Finally, EF<=0.30 was the only gated-SPECT study variable to be associated to a combined clinical event (P=.02).
Cox regression analysis for predicting combined clinical events
Gated-SPECT EF<=0.30 (OR=2.5; 95% CI, 1.2-4.8) was the only independent variable associated to combined clinical events.
Survival curves without events as per gated-SPECT ejection fraction (Figure 3A)
Fig. 3. Kaplan-Meier survival curves without combined clinical events grouped by the presence or absence of severe ventricular dysfunction determined by gated-SPECT. A. Total population analysis. B. Population without clinical restenosis analysis.
The mean survival time free of clinical events was 28 months in patients with ventricular dysfunction (95% CI, 23-32 months), compared with 36 months (95% CI, 33-39 months) in patients with preserved ventricular function (P=.007).
Gated-SPECT determined ventricular function associated with morbidity/mortality in population without restenosis
The prognostic evidence of clinical, angiographic and gated-SPECT variables was analyzed in the percutaneously revascularized population that did not present clinical restenosis during follow-up (n=85). Patients with EF<=0.30 had a significantly shorter survival time (P<.001) and a shorter mean time free of events (P=.001) (Figures 2B and 3B).
DISCUSSION
In the case of a revascularized ischemic heart disease population, gated-SPECT determination of left ventricular EF previous to revascularization has an independent prognostic value. In our study, left ventricular dysfunction confirmed by gated-SPECT (SF<=0.30) was associated to higher morbidity/mortality despite revascularization. Up to our knowledge, our study is the first evaluation of the prognostic value of gated-SPECT performed in a population of revascularized coronary disease patients.
Included population morbidity/mortality during follow-up
Mortality rates in our population were 6.4% to one year. This value is higher than in randomized studies that evaluate revascularization strategy benefits in ischemic heart disease.14,22,23 Such a high mortality is attributable to a higher prevalence of poor prognostic factors in our population sample compared to other studies: a history of previous necrosis in 80% of patients, comorbidity in one third and impaired ventricular function in more than one half.
Left ventricular function prognostic value
EF is a classical prognostic factor for ischemic heart disease patients. Revascularization improves prognosis of patients with left ventricular dysfunction,22,24,25 although ventricular dysfunction will continue to be a risk factor, showing no differences if revascularization was percutaneous or surgical.16,26
Although left ventricular dysfunction is unanimously considered an impaired prognosis indicator, there is no agreement as to EF values that delimit impaired prognosis. In the isotopic ventriculography studies, the cut-off values delimiting impaired prognosis were 0.50,6 0.40,4,10 0.355,8 and 0.309 EF values.
In our population, the gated-SPECT baseline EF<=0.30 previous to revascularization confirmed a five-fold death risk and doubled the combined event risk during follow-up.
An adequate correlation between contrast ventriculography and gated-SPECT for calculating EF has been described.27,28 Despite this correlation, only the gated-SPECT values were associated independently to morbidity/mortality in our study. This gated-SPECT superiority could be attributed to calculating the EF differently than with the contrast ventriculography Dodge method. The first approach uses bead tracing produced by radio-isotopic activity in the myocardial wall, whereas the second method assumes a geometric ellipsoid ventricle, which is not true in some cases.
Baseline myocardial perfusion did not add prognostic value to ventricular function in gated-SPECT
A baseline perfusion score has been used for evaluating myocardial perfusion, with values indicating both severity and extension of the sestamibi captation defects in gated-SPECT. As scan was performed at rest, scores should correspond basically to the extension of the necrotic or severely hypoperfused myocardium. The baseline perfusion score has been associated significantly, although scarcely, with the EF. It has been stated that the infarct size will condition mortality in patients treated with thrombolysis,29 although a relation between the baseline perfusion score and cardiac event appearance was not found in a similar population.30 Our study has not found an association between the baseline perfusion score and deaths or events occurring during follow-up.
The number of asynchronous regions in the 3D left ventricle representation is a gated-SPECT regional wall motion score with similar meaning to the baseline perfusion score, possibly indicating the extension of necrotic or hibernated myocardium caused by severe ischemia. In this case, a closer relation appeared between the asynchronous myocardium extension and EF (r=0.59), and the number of asynchronous regions was associated significantly, but not independently, with mortality.
Limitations
Our study is subject to various limitations. Firstly, the revascularized patients complying with the requirements for gated-SPECT study were not consecutively included, as this depended on scintigraphy camera availability. As the basis of our study is a gated-SPECT protocol using rest scan, there is no information available about induced ischemia extension or myocardial viability, although these variables could have added an independent value to the ventricular function for prognostic prediction in our population. Finally, not all the revascularized population underwent an angiography during follow-up, so the potential effects of asymptomatic restenosis in patient prognosis are unknown.
CONCLUSIONS
Our conclusion is that gated-SPECT studies are a useful tool for predicting prognosis after revascularization of ischemic heart disease patients, on the basis of information they provide on the left ventricular function. Patients with EF¾0.30 obtained by gated-SPECT before revascularization presented a higher risk of death and of combined events of death, non-fatal infarction or hospital re-admission due to cardiac reasons, than patients with a preserved ventricular function, despite revascularization was performed with success.
Correspondence: Dr. Rafael J. Ruiz-Salmerón.
Rúa da Costa, 2, portal 2.o, 5.o B. 36213 Vigo. España.
E-mail: rafael.ruiz.salmeron@sergas.es.
Received 15 April 2002.
Accepted for publication 29 October 2002.