To the Editor,
In patients with severe aortic stenosis and high surgical risk, transcatheter aortic valve implantation is a safe and effective treatment option.1 Two devices are available for this purpose, the CoreValve® from Medtronic and SAPIEN® from Edwards Lifesciences.2
In cases with a poor vascular access (usually patients with a high EuroSCORE, who experience greater mortality and complications), the transapical approach can be used, although it is only available for the SAPIEN® valve.
As an alternative in selected patients who do not have a suitable vascular access, implantation of the CoreValve®3, 4 by direct access through the ascending aorta using a mini-sternotomy or intercostal thoracotomy can be considered. This access was recently described in the treatment of a degenerated bioprosthesis5 and in a case of severe aortic insufficiency 1 week following implantation of a SAPIEN® valve.6
We present 2 cases of severe degenerative aortic stenosis treated with a transcatheter CoreValve® device using direct aortic access and performed by a mixed team of interventional cardiologists and cardiovascular surgeons in 2 Spanish hospitals.
The first patient was an 83 year-old-man with hypertension and chronic atrial fibrillation under anticoagulation. The patient presented with severe aortic stenosis and dysfunction of the left ventricle (left ventricular ejection fraction, 35%), and had been hospitalized several times previously for angina and heart failure. On dobutamine perfusion echocardiography, the peak and mean aortic gradients were 66 mmHg and 35 mmHg, respectively. The calculated valve area was 0.8 cm,2 the aortic ring measured 22 mm, and the ascending aorta, 45 mm, with a very horizontal orientation. Significant coronary disease was not documented. The common femoral arteries had a diameter of <6 mm and the subclavian arteries<4 mm. The logistic EuroSCORE was 35.3%. The patient refused surgical valve replacement.
Following valvuloplasty, a 26-mm CoreValve® aortic prosthesis was implanted by direct aortic access, with a good outcome and no complications. The procedure lasted 230 min. The patient was discharged from hospital on day 8 following surgery. One month later, he was asymptomatic, the peak aortic gradient on echocardiography was 15mmHg, and there was no aortic insufficiency.
The second patient was a 76-year old man, ex-smoker, with diabetes, hypertension, severe chronic obstructive pulmonary disease, severe arterial disease of the supra-aortic trunks (including the subclavian arteries), Leriche syndrome, grade II/IV chronic renal failure, and a central retinal artery embolism. He was admitted to hospital in cardiogenic shock secondary to critical aortic stenosis, and underwent emergency aortic valvuloplasty through a left humeral approach. The coronary arteries showed no significant disease. Left ventricular function was moderately depressed (left ventricular ejection fraction, 42%), and the logistic EuroSCORE was 51.95%.
Once the emergency situation had resolved, the aortic measurements were evaluated: peak and mean gradients following valvuloplasty were 70 mmHg and 42 mmHg; aortic ring was 24 mm and the highly calcified ascending aorta was 36 mm. A 29-mm CoreValve® was successfully implanted using a direct transaortic access, with no complications. The patient was discharged on day 7 following surgery. At 1 month he was asymptomatic, the peak aortic gradient was 18 mmHg, and there was no aortic insufficiency.
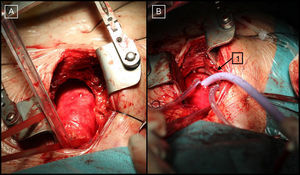
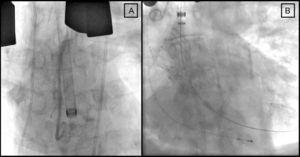
The implantation technique in both patients consisted of a J sternotomy (from the manubrium to the right sternal border at the level of the third or fourth intercostal space) to expose the ascending aorta (Figure 1A). Then, without cardiocirculatory arrest or use of on-pump circulation, a hemostatic suture (“tobacco bag”) was performed at the anterior wall of the ascending aorta, and the aorta was directly punctured with a 9-Fr introducer. Under fluoroscopic guidance, the native valve was crossed and a high support guidewire was placed in the left ventricle. An 18-Fr introducer was advanced along the guidewire (Figs. Figure 1 and Figure 2), the self-expanding CoreValve® device was introduced, and the valve was deployed according to the standard technique1 (Figure 2B).
Figure 1. A: Intraoperative image of ascending aorta exposure. B: Cannulation with an 18-Fr introducer in the ascending aorta (1) (first case).
Figure 2. Fluoroscopy showing the 18-Fr introducer in the ascending aorta before implantation of the valve (A) and correct positioning of the device (B) (second case).
These are the first cases reported in Spain of treatment with a transcatheter CoreValve® system using a direct aortic access. Implantation of the device was possible in both patients, even though neither the femoral nor the subclavian/axillary access could be used. Furthermore, in the patient with a horizontal ascending aorta anatomy, this approach facilitated implantation because of the special angulation adopted by the introducer when the ascending aorta is directly accessed; hence, this characteristic could be of help in similar cases.
We believe that this approach can be used for CoreValve® implantation when a femoral or subclavian/axillary access is not feasible.
Although further experience is needed, the initial safety and efficacy results4 indicate that this technique may be considered a viable alternative to the transapical approach for selected patients such as those presented here.
Corresponding author: cesar.moris@sespa.princast.es