Direct oral anticoagulants have a wide therapeutic window, a predictable anticoagulant effect, and low risk of drug-drug interactions. They are at least as effective as warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) but have a better safety profile, particularly with regard to the risk of intracranial hemorrhage.1
Dronedarone is an antiarrhythmic agent currently indicated for the maintenance of sinus rhythm after successful cardioversion in clinically stable adults with paroxysmal or persistent AF.2 Remarkably, dronedarone significantly reduces the risk of AF recurrence and the incidence of hospitalization due to cardiovascular events or death in patients with paroxysmal or persistent AF.3,4 However, concomitant treatment with dabigatran and dronedarone is contraindicated, since the area under the curve of dabigatran increases by more than 100% with the addition of dronedarone. However, data on the safety of the coadministration of rivaroxaban with dronedarone is lacking and, for this reason, there is no specific recommendation on this issue in the current EHRA Practical Guide on the use of direct oral anticoagulants in NVAF. In fact, in the EHRA guideline, concomitant rivaroxaban and dronedarone use is marked as a “yellow” interaction, with the recommendation to maintain the original dose, unless 2 or more concomitant ‘yellow’ interactions are present.5 The aim of this study was to determine whether there are significant clinical consequences of combining rivaroxaban with dronedarone in NVAF patients.
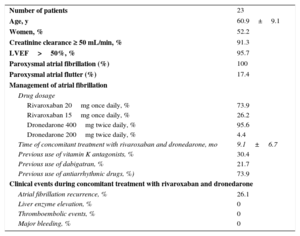
Twenty-three patients with paroxysmal AF (age 60.9±9.1 years) treated with both drugs concomitantly were included in the study (Table). None of the patients had significant structural heart disease except for 1 with mild left ventricular dysfunction without heart failure. About three quarters of the patients were treated with rivaroxaban 20mg once daily and all were treated with dronedarone 400mg twice daily except for 1 treated with dronedarone 200 twice daily due to bradycardia. Most patients had previously taken other antiarrhythmic agents and switched to dronedarone due to AF recurrence or drug intolerance. A total of 30% of patients switched from vitamin K antagonists to rivaroxaban due to lack of adequate international normalized ratio (INR) control or patient preference and 22% switched from dabigatran to rivaroxaban, when dronedarone was initiated. One patient (4.3%) required dronedarone withdrawal due to drug intolerance. At follow-up (9.1±6.7 months), one quarter of the patients had AF recurrence, and there were no thromboembolic or major bleeding events.
Baseline Characteristics and Clinical Events in Patients With Nonvalvular Atrial Fibrillation Treated With Rivaroxaban and Dronedarone
| Number of patients | 23 |
| Age, y | 60.9±9.1 |
| Women, % | 52.2 |
| Creatinine clearance ≥ 50 mL/min, % | 91.3 |
| LVEF>50%, % | 95.7 |
| Paroxysmal atrial fibrillation (%) | 100 |
| Paroxysmal atrial flutter (%) | 17.4 |
| Management of atrial fibrillation | |
| Drug dosage | |
| Rivaroxaban 20mg once daily, % | 73.9 |
| Rivaroxaban 15mg once daily, % | 26.2 |
| Dronedarone 400mg twice daily, % | 95.6 |
| Dronedarone 200mg twice daily, % | 4.4 |
| Time of concomitant treatment with rivaroxaban and dronedarone, mo | 9.1±6.7 |
| Previous use of vitamin K antagonists, % | 30.4 |
| Previous use of dabigatran, % | 21.7 |
| Previous use of antiarrhythmic drugs, %) | 73.9 |
| Clinical events during concomitant treatment with rivaroxaban and dronedarone | |
| Atrial fibrillation recurrence, % | 26.1 |
| Liver enzyme elevation, % | 0 |
| Thromboembolic events, % | 0 |
| Major bleeding, % | 0 |
LVEF, left ventricular ejection fraction.
Dronedarone is a moderate CYP 3A4 inhibitor, a mild CYP 2D6 inhibitor, and a potent P-gp inhibitor. Rivaroxaban is metabolized via CYP3A4, and is a substrate for the P-gp. Therefore, a potential pharmacodynamic interaction could be expected when both drugs are taken concomitantly.6 However, data on the safety of this combination is currently lacking. To our knowledge, this is the first study of the consequences of combining rivaroxaban with dronedarone.
Our study has some limitations. First, a limited number of patients were included. Second, although most patients had normal renal function, one quarter were taking rivaroxaban 15mg one daily. This could have counterbalanced the effect of the increase in the area under the curve of rivaroxaban. Finally, the follow-up was insufficiently long to establish the efficacy and safety of the combination in the long-term.
In conclusion, our work is the first study that provides evidence that the concomitant administration of both drugs is safe and is not associated with significant adverse events. However, these results need to be validated by further studies.
CONFLICTS OF INTERESTC. Escobar has received fees for lectures from Bayer, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi. J. L. López-Sendón and J. L. Merino have received fees for consultancy or lectures from Bayer, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi.