Moderate to torrential tricuspid regurgitation (TR) is estimated to affect 1.6 million people in the United States.1 The classic treatment for TR is optimal medical therapy—mainly diuretics or surgery. However, surgical mortality in isolated tricuspid valve interventions is significantly higher than in any other single valve (∼9%).1 Across the growing range of percutaneous therapeutic alternatives for TR, heterotopic caval valve implantation (CAVI) including Tricento (NVT, Germany) and TricValve (Products&Features, Austria) systems2,3 might be the preferred option when right chamber dilation is more advanced or in patients with prior pacemaker leads. Evaluation of caval anatomy is crucial. Therefore, we aimed to analyze the computed tomography (CT) scans of candidates for this therapy as well as cadaveric models in order to: a) describe main variations of right heart and caval anatomy relevant for CAVI candidates; and b) develop a standardized CT evaluation prior to CAVI procedure.
CT scans from 32 patients with severe to torrential TR eligible for CAVI procedure after exclusion of alternative therapies were centrally analyzed. Images were obtained on a 128-detector row CT scanner (Revolution CT, GE Healthcare, Waukesha, Wisconsin, United States). We tailored the protocol by injecting 75mL of iodixanol (Visipaque 320mg/mL) via an antecubital vein. We manually started the acquisition when the pulmonary artery was completely opacified. Additionally, we prepared a delayed acquisition to be started 70 to 90seconds after contrast material injection (portal phase) to be performed if the inferior vena cava (IVC) and the hepatic veins were not well opacified in the first study. Finally, 3 cadaveric models were used for structural direct analysis. All patients provided informed consent and the study was approved by local ethics committee.
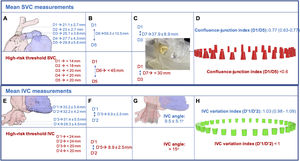
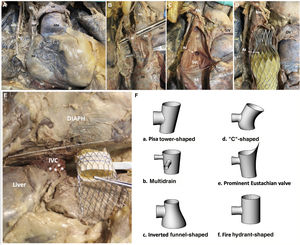
The main measurements and risk thresholds for superior vena cava (SVC) and IVC are summarized in figure 1. The mean cranial-caudal length of SVC was 59.3±10.5 mm potentially leading to mean protrusion into the right atrium of 8 mm, but up to 31.6mm with the self-expanding TricValve device (simulated in figure 1C). In addition, 7 patients (21.9%) showed marked tapering (confluence-junction index <0.6). Both might condition a higher risk of leak and/or valve embolization suggesting the need for higher device implantation. However, this might be associated with a higher risk of azygous vein occlusion during the procedure as identified in the cadaveric model (figure 2C,D). The clinical relevance of this complication is currently unknown. Finally, in 11 patients (34.4%), a pacemaker lead was present.
Superior (A to D) and inferior (E to H vena cava measurements based on computed tomography and suggested thresholds for high-risk of complications. A: confluence between LIV and SVC (D1); SVC at top of PA (D2); SVC at middle of PA (D3); SVC at bottom PA (D4); SVC at RA junction (D5). B: length from D1 to D5 (D6). C: length from D1 to D3 (D7) and example of printed biomodel of a short and tapered SVC. D: confluence-junction index and different SVC morphologies according to this index and D7. E: diameter at the confluence between IVC and RA transition (D’1); IVC at top of HV (D’2); IVC at the bottom of HV (D’3); 5cm below LIV-RA transition (D’4). F: length from D’1 to D’2 (D’5). G: simulation of the angle determined by lower and upper segments of IVC on TricValve final position. H: IVC variation index and different IVC morphologies according to this index. HV, hepatic veins; IVC, inferior vena cava; LIV, left innominate vein; PA, pulmonary artery; RA, right atrium; SVC, superior vena cava.
Cadaveric model of SVC (A to D) and IVC (E) and schematic potential shapes of IVC (F). Celiac plexus fibers (asterisk). AAo, arteria aorta; Az, azygos vein; DIAPH, diaphragm; IVC, inferior vena cava; LIV, left innominate vein; RA, right atrium; RIV, right innominate vein; RPN, right phrenic nerve; SVC, superior vena cava.
The mean distance from IVC to upper part of hepatic veins was 8.9±2.5 mm, potentially excluding 30 patients (93.75%) from receiving a self-expanding Tricento device whose current limit is 12 mm. However, in none of them was this distance prohibitive for TricValve. In addition, the angle determined by the IVC segments above and below the hepatic veins (mean: 9.5±5.1°) widely varied (range: 4.4°-18.4°) and might influence the final landing zone and the risk of valve migration and residual leak (figure 1G). The presence of celiac plexus fibers surrounding the IVC was identified in the cadaveric model (figure 2E) and might explain the common presence of temporary radiated pain often detected in the next few hours after IVC prosthesis implant due to overexpansion. Several morphological patterns of IVC were identified and have been schematically depicted in figure 1H and figure 2). There was wide variability in the length of the Eustachian valve, leading to poorly contrasted IVC despite repeat CT in 3 patients (9.4%), suggesting a greater value of echocardiography for IVC prosthesis sizing if a long Eustachian valve is present.
Preliminary positive results with dedicated devices have promoted high expectations on the results of the ongoing TRICUS study (NCT04141137). Our results highlight the relevance of CT measurements (above other imaging techniques) for optimal procedural planning. Moreover, the success of CAVI alternatives lies not only in the device chosen, but also in correct identification of relevant parameters of the anatomy and physiology to predict the risk of complications and efficacy, respectively.
In conclusion, according to our imaging analysis, TricValve might be preferred over Tricento in a large proportion of cases due to short distance to hepatic veins (HV) but Tricento could be a better alternative in patients with excessive tapering of SVC or marked angulation of the IVC as both increase the risk for valve leakage or embolization.
FundingNone to declare.
Conflicts of interestI.J. Amat-Santos is proctor for Products & Features.