The cardiac manifestations of influenza A virus infection are currently not well defined. Infection with influenza A virus is estimated to cause myocardial injury in 10% of patients, and the reported manifestations are diverse, from asymptomatic through to acute myocarditis,1 tako-tsubo syndrome, and cardiac tamponade.2
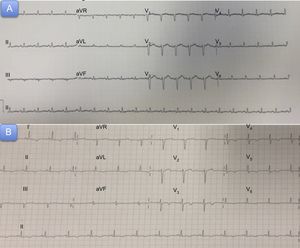
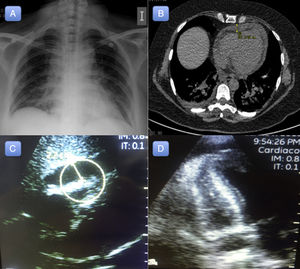
A 54-year-old woman with a history of diverticulitis and primary biliary cirrhosis attended the emergency department with generalized weakness and muscle pain. The patient reported having had cold symptoms and fever (39°C) during the previous week. The physical examination and hemodynamic profile on admission were normal. An initial electrocardiogram revealed sinus tachycardia without repolarization abnormalities (Figure 1). A chest X-ray revealed cardiomegaly with signs of fluid overload (Figure 2A), and blood analysis revealed mild leukocytosis. Peak high-sensitivity troponin T was 312 ng/L, C-reactive protein was elevated (15 mg/L), and procalcitonin was normal.
The patient's clinical course while in the emergency department was poor, with onset of respiratory failure and lactic acidosis (lactic acid, 4.2 mmol/L) associated with abdominal pain. Suspected sepsis was investigated with a chest and abdomen computed tomography scan, which revealed signs of congestive heart failure and a small pericardial effusion (maximum diameter, 12 mm) (Figure 2B). Transthoracic echocardiography identified moderate left ventricular dysfunction (ejection fraction 37% by the Simpson method) and a small pericardial effusion. Over a period of hours, the patient progressively developed overt hemodynamic instability that progressed to cardiogenic shock, requiring orotracheal intubation, invasive mechanical ventilation, and vasoactive and inotropic support with norepinephrine up to 0.8 μg/kg/min and dobutamine up to 10 μg/kg/min. Despite these measures, the hemodynamic profile continued to deteriorate, with increasing tachycardia, frank hypotension, and oliguric acute kidney failure. A second transthoracic echocardiogram (8hours after the first) revealed significant enlargement of the pericardial effusion, which was now severe (22 mm) (Figure 2C and 2D), and signs of cardiac tamponade.
Emergency pericardiocentesis removed 300 mL of serous fluid. After this procedure, the patient's hemodynamic profile improved immediately, and vasoactive support was significantly reduced. A pericardial drainage catheter was fitted and extracted abundant fluid (50 mL/h over the following 4hours). Over the next 24hours, the patient's hemodynamic profile again deteriorated, with worsening hypotension and lactic acidosis, requiring an increase in norepinephrine support to 0.55 μg/kg/min. A new transthoracic echocardiogram showed almost complete resolution of the pericardial effusion and persistence of the ventricular dysfunction. The pericardial fluid was negative for bacterial cultures, acid-fast bacilli, and malignancy. An influenza A test was positive for the H1N3 strain.
The total volume of drained pericardial fluid was around 600mL. A control echocardiogram at 72 hours showed recovered ventricular function and an absence of pericardial effusion, and the drainage catheter was removed. The patient was discharged in a stable condition.
In this report, we present a case of cardiogenic shock and cardiac tamponade in a patient with acute myopericarditis caused by influenza A H1N3 infection. Influenza A virus has broad cardiac manifestations that typically appear 4 to 9 days after the onset of the first symptoms of infection. Cardiac involvement predominantly affects women, and there is wide variability in the age of patients presenting with this condition.3 Cardiac manifestations tend to be mild4; however, there have been reports of major cardiac injury, ranging from acute pericarditis to fulminant myocarditis and cardiac tamponade.2
The patient presented here had influenza symptoms in the week before admission. Although her condition was stable on admission and the pericardial effusion was initially small, this patient's case was remarkable due to the rapid development (in under 8hours) of cardiac tamponade and cardiogenic shock, with approximately 500 mL of fluid drained within 3hours of the pericardiocentesis. The patient was not treated with anti-inflammatory drugs due to her deteriorating renal function, reflected in a creatine level of 3.78 mg/dL.
Half of patients infected with influenza virus show electrocardiographic alterations, which can include fatal arrhythmias.5 Some studies have reported effects on ventricular contractility in 70% to 80% of patients.6 Several reports have identified a greater predisposition to hemodynamic instability and severe pericardial effusion among patients infected with influenza A.
There is no consensus on the optimal treatment for pericardial effusion caused by H1N3 infection; however, pericardiocentesis is the first choice treatment in situations of hemodynamic instability. Although the evidence is not clear, the recommended treatment for patients with fulminant myocarditis is neuraminidase inhibitor therapy and mechanical respiratory support if considered necessary. Evidence is similarly lacking for the efficacy of treating this condition with nonsteroidal anti-inflammatory drugs or colchicine; however, these treatments could prevent recurrence in the short-term. We present a case of cardiac tamponade in a patient with no known history of heart disease and who had severe influenza A virus infection and a predisposition to rapid establishment of significant pericardial effusion and hemodynamic instability.