Keywords
INTRODUCTION
Until the 1990s, myectomy was the treatment of choice for reducing obstruction in patients with hypertrophic obstructive cardiomyopathy and symptoms refractory to drug therapy.1 The therapeutic options increased with the introduction of DDD ventricular pacing2 and more recently, percutaneous septal ablation.3-7 Because of its effectiveness and safety, the use of this last option has become increasingly more widespread.8,9 Nevertheless, complications or undesirable effects can occur on occasion.10-14 We describe for the first time a complication associated with this technique consisting of rupture of the occlusion balloon and extravasation of alcohol toward the left anterior descending artery.
CASE STUDY
A 57-year-old man diagnosed with hypertrophic obstructive cardiomyopathy and currently on beta-blockers, verapamil, and furosemide with aggravation of his symptoms over the past year, presenting with dyspnea on slight exertion and acute pulmonary edema that required hospitalization. The echocardiogram showed severe left ventricle hypertrophy affecting the septum (27 mm), systolic anterior motion of the mitral leaflet, and a maximum gradient of 116 mm Hg in the left ventricular outflow tract. A decision was made to perform percutaneous septal ablation.
The procedure was performed using the technique described by Seggewiss et al.3,4 Following prophylactic placement of an intracardiac pacemaker, the ostium of the left coronary was canalized with a 7 French Judkins left 4.0 guiding catheter. Selective catheterization of the first septal artery was done using an ACS BMW intracoronary guidewire (0.014x190). Septal artery adequacy was studied by echocardiography with 1 mL of contrast material (Levovist®, 350 mg/mL) (Figure 1). The diameter of the target vessel was 2.3 mm; a 2.5x9-mm Maverick® balloon was advanced and inflated to 7 atmospheres. After confirming the absence of reflux toward the distal territory of the left anterior descending artery, 2 mL of 96% alcohol were injected. The balloon remained inflated for 10 min, after which time a persistent gradient of 100 mm Hg was observed; therefore, a second 2-mL dose of alcohol was injected. The balloon ruptured immediately after the injection was started and part of the alcohol passed toward the left coronary vasculature, producing severe anginal pain. The echocardiogram showed "illumination" of the entire left ventricular endocardium, including the lateral wall (Figure 1C). Left ventricular contractility deteriorated and the ejection fraction dropped from 65% to 50%. This was accompanied by atrioventricular block (AV) with accelerated idioventricular rhythm that required ventricular pacing, and a maximum creatine kinase peak of 526 U/L. On the third day, some advanced AV block episodes persisted, left ventricle contractility was normal, and the gradient was >100 mm Hg. On the fifth day of hospitalization, a permanent DDDR pacemaker was implanted and the gradient was reduced to 35 mm Hg (Figure 2).
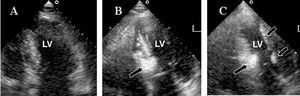
Figure 1. A: 2-D image of basal left ventricle (A). B: following injection of contrast material in the first septal branch, the first third of the interventricular septum is opacified. C: contrast material reflux to the coronary vasculature "illuminates" the entire endocardium, including the lateral wall.
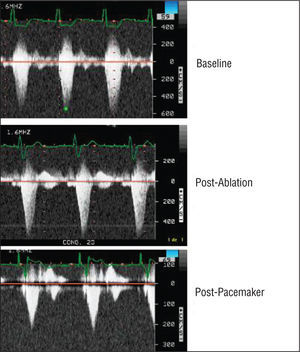
Figure 2. Evolution of the left ventricular outflow tract gradient (baseline, 135 mm Hg). After ablation, the electrocardiogram shows widening due to temporary pacing, with a gradient of 120 mm Hg. The gradient dropped to 35 mm Hg following DDD pacing.
DISCUSSION
Following Sigwar's description7 in 1995 to date, more than 2000 patients have been treated by septal alcohol ablation, with immediate success achieved in 90%.9-14 Complications of the procedure include a 24% in-hospital mortality rate9 and intraventricular conduction disorders that are often transient, but that require implantation of a permanent pacemaker in 13% to 22% of patients.13 Other, less frequent complications include ventricular arrhythmia, left anterior descending artery injury caused by occlusion or dissection,11 and alcohol reflux toward this artery.14 Balloon rupture during the procedure has not been previously reported.
Our patient met the ideal requirements for this procedure: functional class III/IV, septal thickness >18 mm and gradient >30 mm Hg at rest.3,4 Balloon rupture caused alcohol reflux toward the territory of the left anterior artery, but fortunately had no severe consequences, only mild, transient overall left ventricular hypokinesia and AV blockade that required pacing. We are unaware of the potential mechanisms that could cause rupture of the balloon. Contractility recovered rapidly and enzyme elevation was minimal. In successful procedures described in the literature, creatine kinase levels of 300 to 2000 U/L have been reported. Several cases of alcohol reflux towards the left anterior artery due to poor balloon positioning (too proximal to the vessel outlet) or after a second injection of alcohol have been described. ST segment elevation, ventricular arrhythmia, and high enzyme elevation (1496 U) are observed in these cases.14 In our patient, the control echocardiogram showed that alcohol had passed into the myocardium (increased endocardial echogenicity), and the procedure was stopped prematurely, possibly limiting the resulting damage. Alcohol reinjection must be done with great care, since necrosis caused by the first reinjection can lead to increased coronary resistance and facilitate alcohol reflux.14
Correspondence: Dr. G. de la Morena Valenzuela.
Servicio de Cardiología. Hospital Universitario Virgen de la Arrixaca.
Ctra. Madrid-Cartagena, s/n. 30120 Murcia. España.
E-mail: gdlmorena@yahoo.es