In orthotopic heart transplant, the biatrial anastomosis technique introduced by Lower and Shumway is widely used.1 This technique distorts the right atrial geometry and hemodynamics, leading to increased mean atrial pressure, tricuspid and mitral regurgitation, electrical asynchrony, and an increased incidence of atrial flutter.2 In the literature, the most commonly described type is cavotricuspid isthmus-dependent flutter, in which the posterior barrier of the circuit is the atrioatrial suture line rather than the crista terminalis. However, when there is electrical conduction between the recipient atrium (AR) and the donor atrium (AD) across the atrial anastomosis, either by electrotonic transmission mechanisms or by direct conduction,3 the AR can contribute to the development of arrhythmias in the donor heart.4
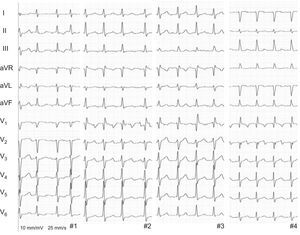
We present 4 patients who underwent orthotopic heart transplant with the biatrial anastomosis technique and developed atypical atrial flutter long after surgery (median, 5.5 years [range, 3–10 years]). All reported palpitations, with no signs or symptoms of heart failure, and electrocardiogram showed atrial flutter with variable atrioventricular conduction (Figure 1). Once rejection had been excluded as an acute cause, a guided ablation procedure was performed with endomyocardial biopsy using a 3-dimensional navigation system (CARTO: ThermoCool SmartTouch ablation catheter, Biosense Webster).
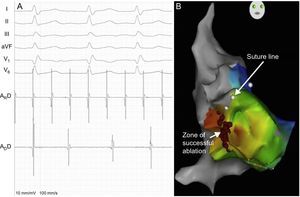
In all patients, endocardial mapping showed a macroreentrant tachycardia with a variable relationship between the AR and AD. There was a frequency gradient from the AR, which had a shorter, regular cycle length, to the AD, which had a longer cycle length (Figure 2A). The AD cycle length was also variable in 2 patients, with a Wenckebach-type periodicity. Guided by the presence of double potentials, we proceeded to anatomical delineation of the biatrial suture (AR-AD) and performed activation mapping of the AD to determine early activation sites around the suture (Figure 2B). In 3 patients, an earliest activation site was found in the lateral wall of the right atrium (9-10 o’clock position of the suture line in an anteroposterior view), and in 1 patient, 2 earliest activation sites were found (at the 10 o’clock and 12 o’clock positions). AR entrainment maneuvers demonstrated an increased variability and prolonged mean cycle length in the AD in all patients and short return cycles in the right AR in 1 patient. Electrocardiograms of the earliest activation sites in the right AD showed features of atrioatrial continuity; radiofrequency ablation of these areas achieved AR-AD electrical dissociation. Thereafter, the AR remained in atrial flutter, and the AD in sinus rhythm, and the palpitations resolved. One patient underwent ablation of a macroreentrant tachycardia in the right AR; in the other 3 patients, long return cycles were confirmed and, with the suspicion of a left AR origin, sinus rhythm was restored by overstimulation.
A: intracardiac recordings (ARD, distal catheter dipole in recipient atrium; ADD, distal catheter dipole in donor atrium) and electrocardiographic tracings of macroreentrant tachycardia. B: right anterior oblique image of the electroanatomical reconstruction and activation map of the donor atrium, showing the surgical suture line (white dots) and the radiofrequency ablation zone in the anterolateral right atrium (red dots).
As a general rule, atrial fibrillation is common in the immediate posttransplant period, whereas macroreentrant tachycardia develops in the longer term and is predominantly cavotricuspid isthmus dependent.5 However, some series have described a similar incidence of atypical flutter with an AR origin and transmission to the AD via atrioatrial connections.6 Electrocardiographic diagnosis is often complicated: distorted electrical propagation in the atria means that F waves are not generated, even in the common types of atrial flutter.6 Therefore, in electrophysiological studies, electrical conduction between the AR and the AD should be included as part of a systemized study that also includes: a) analysis of frequency gradients; b) entrainment maneuvers in the cavotricuspid isthmus, and c) entrainment maneuvers in the right AR.
The optimum treatment strategy for atypical forms of atrial flutter is not fully established. As our series demonstrates, ablation of atrioatrial connections at the surgical suture line can be enough to terminate macroreentrant tachycardia in the AD and its associated symptoms, namely palpitations in these patients. However, there remain some unknowns regarding persistence of the tachycardia in the AR, including the embolic risk conferred to patients. The ideal strategy would be ablation of the slow conduction isthmus of the macroreentrant tachycardia, located in the AR. This was only possible in 1 (25%) of our patients, who had a right AR origin. The other patients had signs of a left AR origin, which would have required a transseptal puncture; these patients were treated conservatively. No patients received anticoagulant therapy during follow-up (54, 35, 5, and 4 months, respectively), and they made good clinical progress free from symptoms and complications, with sinus rhythm on electrocardiogram.