Atrial fibrillation (AF) is the most common cardiac arrhythmia, and its prevalence is increasing in our setting.1 AF is one of the main causes of stroke, heart failure cardiac morbidity, and sudden cardiac death. Although the treatment is mainly pharmacological, percutaneous and surgical ablation techniques are also used for this purpose.
We present the case of a patient who underwent radiofrequency ablation of AF, with a fatal outcome. A 61-year-old woman with Fabry disease had renal (proteinuria), ocular (cornea verticillata), and cardiac involvement (persistent AF, asymmetrical left ventricular hypertrophy, progressive left ventricular dysfunction at 40% in the last determination, and atrioventricular conduction defects requiring implantation of a dual-chamber pacemaker). She was receiving enzyme replacement therapy with agalsidase alfa twice weekly, candesartan, bisoprolol, furosemide, spironolactone, and apixaban. The patient had recurrent AF episodes with poor hemodynamic tolerance, which had been treated with electric cardioversion on several occasions, but with early relapses. Attempts were made to maintain sinus rhythm with amiodarone, but she developed drug-related hepatotoxicity, which led to permanent withdrawal of this treatment. In light of the patient's symptoms, a strategy of percutaneous radiofrequency ablation was contemplated to control the arrhythmia. The procedure was performed with a TactiCath Quartz 75 irrigated catheter with a contact force sensor (St Jude Medical). Electrical isolation of the pulmonary veins was carried out. As the patient had persistent AF with several relapses, in addition to associated structural heart disease with considerable left atrial dilatation, ablation lines were performed in the posterior wall of the left atrium and mitral isthmus, as well as ablation of complex fragmented atrial electrograms. Radiofrequency applications lasted <15 s with a contact force of < 10g and power of ≤ 25W, with the aim of increasing the safety of the procedure.
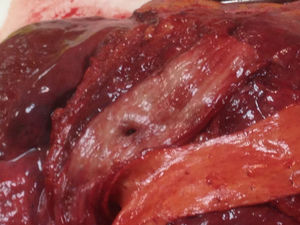
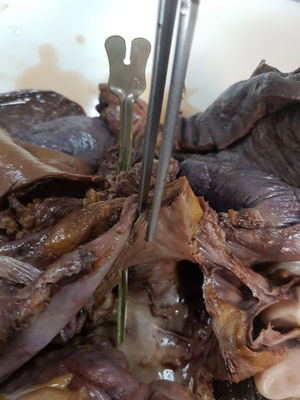
At 18 days following the procedure, the patient was admitted for a new episode of heart failure with recurrent AF. During hospitalization, she experienced sudden onset of hemiparesis and facial paralysis. Cranial computed tomography was performed to investigate the presence of an intracranial hematoma, with normal findings. Suddenly, she showed frank hematemesis with hemodynamic instability, which required stabilization. Emergent endoscopy of the upper gastrointestinal tract was performed to rule out gastrointestinal bleeding related to the oral anticoagulation. Clot remnants were seen in the gastric fundus, and during withdrawal of the endoscope, an image similar to that of a pulsatile diverticulum, was observed in the middle third of the esophagus. Immediately, and coinciding with air insufflation, the patient experienced ventricular fibrillation refractory to advanced cardiopulmonary resuscitation maneuvers and died. An atrioesophageal fistula secondary to AF ablation was strongly suspected. Clinical autopsy confirmed the diagnosis (Figures 1 and 2), and provided macroscopic data consistent with cardiac involvement due to Fabry disease.
In our patient, the presumed pathophysiology underlying the neurological symptoms was an embolism caused by an air bubble or food content passing from the esophagus to the left atrium and deposited in the brain tissue through the systemic circulation. The gastrointestinal bleeding can be explained by passage of blood from the left atrium to a low-pressure chamber, like the esophagus, and the ventricular fibrillation by passage of insufflated air from the esophagus to the left ventricle during gastrointestinal endoscopy.
Electrical isolation of pulmonary veins is an effective, widely used technique to treat AF. Radiofrequency energy or cryotherapy is used to electrically isolate the atrial tissue implicated in generating or sustaining the arrhythmia.2 Additional ablation lines can be applied in the left atrial wall when there is structural heart disease, as was seen in our patient.
This procedure has a class I recommendation in clinical practice guidelines3 for symptomatic paroxysmal AF failing antiarrhythmic treatment, and a class IIa indication in some cases of persistent symptomatic AF failing treatment.
Although the technique is quite safe, it is not free from complications. The mortality rate is estimated at less than 2 deaths per 1000 procedures.3 In Spain, 2953 procedures were indicated in 2016, with an acceptable technique-associated complication rate (3.9%) and no reported deaths.4 Complications, although infrequent, are potentially fatal, including cardiac tamponade, stroke, and atrial-esophageal fistula, among others. An experienced team, together with adequate patient selection, significantly reduces the incidence of these complications.5 In addition, a recent study has related the CHA2DS2-VASc score with the risk of periprocedure complications.4
Atrioesophageal fistula is an uncommon complication (< 1 per 1000 patients)2 that can develop between 3 days and 5 weeks following ablation. It should be promptly suspected when there is onset of fever, chest pain, dysphagia, gastrointestinal tract bleeding neurological symptoms, or sepsis. Associated mortality is around 100% when untreated and 32% when prompt surgery is performed.6