Keywords
INTRODUCTION
For more than 4 decades surgical myocardial revascularization has proven to be one of the most effective and long-lasting therapies in the treatment of ischemic heart disease (IHD), especially in more complex anatomies. This procedure, which was initially based on coronary artery bypass grafting (CABG) using saphenous vein (SV) grafts, spectacularly improved the clinical outcome when the internal mammary artery (IMA) or internal thoracic artery started to be used to revascularize the left anterior descending artery (LAD). This benefit is maintained due to the excellent long-term patency of the IMA as a consequence of its resistance to histological changes that jeopardize the patency of venous grafts in the short- and mid-term: i.e. fibrous intimal hyperplasia and atherosclerosis.
These results encouraged surgeons to use this artery in territories different from the LAD, and also to employ the right IMA and other arterial conduits. Despite all the experience built up over more than 3 decades, only the use of the left IMA to revascularize the LAD remains undisputed in any CABG intervention. However, the use of the IMA in other arteries, the advantages of using the second IMA, and the indications and results of other types of arterial grafts are still widely debated.
Based on the most recent publications, this article discusses the characteristics of the different types of arterial conduits, the different techniques available, and their clinical and angiographic outcomes. In addition, the advantages and limitations of revascularization via arterial conduits in different anatomical and clinical settings in IHD are analyzed.
BACKGROUND
The first attempts at revascularization of the myocardium via non-native cardiac arteries date back to the 1940s, long before CABG was established as a therapeutic strategy in IHD. A Canadian surgeon, Arthur Vineberg,1 developed different experimental techniques to perfuse the myocardium via the IMA and in 1958 published the first clinical results of intramyocardial implantation of this artery. Years later, Effler et al2 demonstrated that this type of implant could remain patent for years, creating links with the coronary arterioles and helping to improve myocardial perfusion. These results inspired other groups to develop techniques that made it possible to increase coronary flow by directly connecting the IMA to the coronary circulation. In 1964, Spencer et al3 published the first results of mammary artery-coronary artery anastomosis under cardiopulmonary bypass (CPB) and, a few years later, Kolesov,4 reported his initial experience with beating heart mammary artery-coronary artery anastomosis.
However, the great difficulty of anastomosis with the IMA meant that its use was relatively restricted up to the mid-1980s in favor of SV grafting, which is more adaptable and easier to manage. Only the continued work of some pioneers made it possible to demonstrate the greater long-term patency of the IMA grafts and the clinical advantages offered by this. The work of George Green5 deserves special mention as he was one of the few surgeons that continued using the IMA in the 1970s and whose excellent results over 15 years were published in 1986. Encouraged by these results, Kay et al6 began to use both IMA, while Loop et al7 introduced the free graft, disconnecting the IMA from the subclavian artery and anastomizing it proximally to the ascending aorta to revascularize coronary arteries which the IMA could not reach in situ. In the same line, Tector et al8 introduced sequential IMA grafts, carrying out one or more side-to-side anastomoses to revascularize more than one coronary branch with the same graft.
As the use of the IMA became more general, other arterial conduits were introduced as an alternative or a complement that enabled the revascularization of arterial territories different to the LAD. In 1973, Carpentier et al9 began to use the radial artery (RA) as a graft that, due to its greater caliber and wall thickness, seemed easier to employ as a free conduit. Meanwhile, Edwards et al10 used the splenic artery for revascularizing the lower part of the heart. Nevertheless, both types of graft were soon abandoned because of the poor angiographic outcomes. Several years later, Pym et al11 described the technique for using the right gastroepiploic artery (GEA) with the same aim and with better results.
Other arteries, such as the inferior epigastric, ulnar, and subscapular arteries have also been used in an attempt to obtain complete revascularization with arterial grafts.
TYPES OF ARTERIAL GRAFTS
Classification
The arterial segments used as coronary conduits are conduit arteries and, as such, share certain histological and histochemical characteristics that differentiate them clearly from the SV. However, their biological properties are not uniform; they have a different embryological origin and anatomical structure. Their reactivity also differs, whether spontaneous or induced by various pharmacological agents. These differences can have an impact on perioperative behavior and long-term patency. He and Yang12 proposed a classification of arterial conduits based on embryological origin, histological structures and reactivity that has been widely accepted (Table).
Anatomical and Histological Characteristics
The IMA is the first descending branch of the subclavian artery and has a trajectory parallel to the sternum making access easy via median sternotomy. It has a caliber of around 2 mm which is very similar to that of coronary arteries. The intimal layer is thin for most of its length and is separated from the media by a well-defined internal elastic lamina with few fenestrations. The wall is around 350-µm thick and is basically made up of an elastic lamina which alternates with smooth muscle fibers at its proximal and distal ends.13
The RA has a caliber somewhat larger than the IMA and is long enough to reach practically any coronary artery. Unlike the latter, its medial layer is much thicker, close to 500 µm, and is basically made up of smooth muscle fibers.13 Furthermore, its internal elastic membrane has multiple fenestrations and invariably presents mild or moderate intimal hyperplasia.
The right GEA is a branch of the gastroduodenal artery that passes alongside the greater curve of the stomach anastomizing with the left GEA, which is a branch of the splenic artery. Realistically, around the first 20 cm of the artery only can be used. The GEA has a caliber and length similar to those of the RA; >2.5 mm at its source and around 1.8 mm at 15 cm from this point. Its medial layer is also basically muscle fiber, although somewhat less thick than that of the RA. Like the latter, its internal elastic lamina has many fenestrations, although it tends to have a smaller degree of intimal hyperplasia.14
The inferior epigastric artery (IEA) is a rising branch of the external ileac artery, easily approachable from behind the lower part of the anterior abdominal straight muscle. Its length and thickness vary, although a useable segment between 10 cm and 12 cm can be found in most patients. It has a smaller caliber than most arterial grafts, especially further from its source. Its histological structure is very similar to that of the RA, although the thickness of the medial layer is also smaller and fluctuates between 160 µm and 300 µm.14 It also has a moderately hyperplastic intimal layer.
The vasa vasorum does not pass through the adventitia in any of these arteries which are nourished by diffusion from the arterial lumen. This means that their viability is not compromised when they are disconnected from their artery of origin and used as free grafts.
Endothelial Function and Muscle Tone
In addition to acting as a fundamental element in the prevention of platelet clustering and the development of atherosclerotic lesions, the arterial endothelium plays a determining role in modulating vascular tone via the release of vasoactive substances. This interaction between the endothelium and the smooth muscle fibers is fundamental to the capacity of arterial grafts to adapt flow to myocardial demand and is one of the reasons for their excellent long-term patency. Although the capacity of the different arteries to liberate vasodilators is not the same, these differences tend to be small and their capacity to do so is much higher than that of the SV.15 In addition, marked differences have not been found in their capacity to relax independently of the endothelium. On the other hand, the endothelium also generates vasoconstrictors, such as thromboxane A2 and endothelin, and there are receptors for different vasoconstrictors in the muscular layer of the arteries. The vasoconstrictive response of each artery to the various agents also differs regarding sensitivity and magnitude. In general, type II and III arteries, which have a more developed muscular layer, have a more intense vasoconstrictive response to any stimulus in vitro,16 which is fully matched by their behavior in vivo. The RA, specifically, has a contractile capacity higher than the IMA and even the GEA.17
INTERNAL MAMMARY ARTERY
The location, caliber, exceptional histological structure, and excellent endothelial function have made the IMA the graft of choice for CABG.
Technical Options
Either of the 2 IMA can be used while maintaining their origin in the subclavian artery (in situ graft) or by disconnecting them from this and anastomizing them to the aorta or the body of another arterial or venous graft (free graft). On the other hand, as with any other type of graft, the IMA can be used for revascularizing a single vessel or several coronary branches via one or more side-to-side anastomoses (sequential grafts). Thus, the need to use venous grafts is reduced and manipulation of the aorta is lessened or eliminated entirely. On the other hand, the IMA can be harvested together with its satellite veins, the attached part of the intercostal muscles and the intrathoracic fascia (pedicled graft) or without any type of accompanying tissue (skeletonized graft). Skeletonization makes a longer conduit possible and helps the artery to expand, due to denervation, thus enabling side-to-side anastomoses.18 The initial flow of the skeletonized IMA is also greater than when harvested as a pedicle.19 In addition, this technique is less harmful for the thoracic wall and decreases the chances of accidentally puncturing the pleura.20 Above all, skeletonization preserves the vascularization of the sternum more effectively by maintaining the links between the internal branches, which supply the sternum, and the anterior intercostal arteries that, in turn, connect to the posterior intercostal arteries from the thoracic aorta.21 However, skeletonization is technically more demanding, entails a greater probability of damaging the artery during harvesting, and deprives it of the vasa vasorum and venous and lymphatic drainage. Although it has not been demonstrated that skeletonization per se changes structural integrity, endothelial function, and the reactivity of the IMA, its effects on long-term patency remain unknown.22
The in situ left IMA is preferably used to revascularize the LAD and/or its diagonal branches, either as a simple or sequential graft. In the few surgical patients where revascularizing the LAD is unnecessary, the left IMA tends to be targeted at the most important coronary artery, usually a branch of the circumflex artery (LCX), either in situ or as a free graft. The left IMA is used as a free graft when there is a stenosis at the origin of the subclavian artery or, less frequently, to take advantage of arteries injured during dissection of its proximal part or to revascularize coronary branches unreachable in its in situ location23
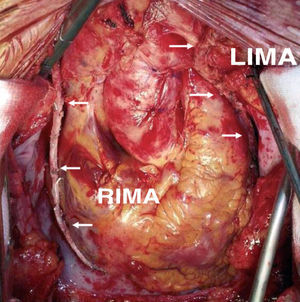
The in situ right IMA can also be used to revascularize branches of the left coronary artery, by either reaching the LAD from in front of the heart or a branch of the LCX from behind the great vessels. In the first case, the IMA crosses the midline over the aorta, which involves a serious drawback in case a new intervention is required. When passed across the transverse sinus it is difficult to reach the more distal branches of the LCX. On the other hand, for the purpose of hemostasis or checking its orientation, the artery remains inaccessible over most of this trajectory, and would be vulnerable during dissection of the aorta in any further intervention.24 Thus, the customary practice is to target the in situ right IMA to the medial or distal right coronary artery (RCA) or, if long enough, to the posterior interventricular artery (PDA) (Figure 1). The former vessel usually has a greater caliber and thicker wall than the IMA, making anastomosis difficult. Furthermore, coronary disease often progresses in the segment between the acute margin and crux cordis, thus compromising the outcome of the grafts located before this segment.25 On the other hand, when the IMA is used to revascularize the PDA, it is often necessary to use its most distal segment, which has a smaller caliber and a greater tendency to spasm.
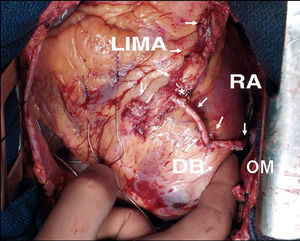
Figure 1. Revascularization with both mammary arteries in situ, using the pedicled left mammary artery to the anterior descending artery and the skeletonized free right mammary artery to the distal right coronary artery. LIMA indicates left internal mammary artery; RIMA, right internal mammary artery.
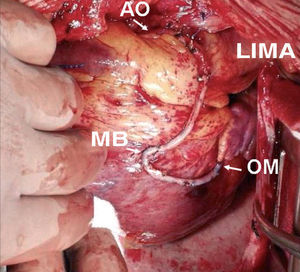
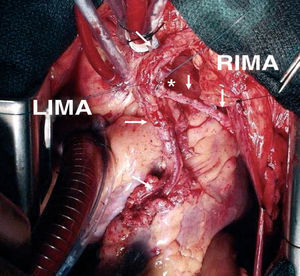
The use of the right IMA as a free graft makes it possible to reach more distant coronary vessels and eliminates some of the mentioned drawbacks of the in situ IMA (Figure 2). However, anastomosis between the aorta and IMA is technically complex due to its small caliber and the different thickness of both vessels. Alternatively, the IMA can be anastomized to the origin of a venous graft or to the body of a left IMA graft26 (Figure 3). In many patients, complete revascularization can be achieved with the exclusive use of the 2 IMA with this inverted Y or T arrangement and one or more sequential anastomoses when needed.
Figure 2. Sequential skeletonized free right internal mammary artery graft to a medium branch and the obtuse marginal artery. RIMA indicates right internal mammary artery; AO, anastomosis to ascending aorta; MB, side-to-side anastomosis to the medium branch; OM, end-to-side anastomosis to obtuse marginal artery.
Figure 3. Composite T-graft with in situ pedicled left mammary artery to the anterior descending artery, and skeletonized free right internal mammary artery to a marginal branch. LIMA indicates left internal mammary artery; RIMA, right internal mammary artery. *End-to-side anastomosis between the internal mammary arteries.
Patency
The patency of the IMA depends on the target artery, severity of stenosis and the quality of its distal bed. The patency of the in situ left IMA anastomized to the LAD is 95% after 1 year. Later occlusions are exceptional such that at 20 years m ore than 90% are still patent.27 Patency is somewhat lower when it is targeted at other arteries. Tatoulis et al,28 studied 15-year patency in over 2000 IMA grafts. It decreased from 97% in the grafts targeted at the LAD, to 91% and 84% in those targeted at the LCX and RCA, respectively.
Although the lower long-term patency of the right IMA has been questioned, recent studies have demonstrated that, when the revascularized vessel is taken into account, patency is equivalent to that of the left IMA. In the aforementioned study, the patency of the in situ left and right IMA used for the LAD was 97% and of 95%, respectively, at 15 years.28 As with other arterial conduits, the patency of the in situ right IMA is also lower when it is targeted at the RCA.29
Data published on free IMA patency basically refer to the right IMA. Only a slight reduction in patency is involved, but this is justified by the different types of target artery. Thus, in a series of 1454 patients with double IMA grafts where the right IMA was used as a free graft for the left territory in 70% of cases, patency at 5 years was slight, but not significantly lower than in situ left IMA (89% vs 96%).30
There is little information available regarding the patency of skeletonized IMA. In a study comparing the clinical and angiographic results of this type of graft with those of pedicled IMA, Calafiore et al31 found that short- and mid-term patency were similar, although control angiography was only done in 19% of the patients. Hirose et al32 obtained similar results in a large number of diabetic patients revascularized with both techniques.
The side-to-side anastomoses of sequential grafts, although technically more demanding, have excellent patency depending on technical expertise. Angiographic follow-up done in the initial months found anastomosis patency to be more than 95%.33
On the other hand, it has been found that the long-term patency of the IMA is greater when the degree of stenosis in the target artery is >60%, especially in the case of the right IMA (patency 65% with <60% stenosis, and 97% with more stenosis).28 However, in a recent study with more than 2000 patients, researchers at the Cleveland Clinic found that IMA patency decreases only slightly when the degree of stenosis in the coronary artery is less, and that there seems to be no degree of stenosis below which patency is significantly reduced.34
Clinical Results
As expected, the excellent long-term patency of the IMA offers clear clinical benefits. Although not supported by randomized studies, the advantages of revascularizing the LAD with the IMA are indisputable and this strategy is regarded as a fundamental element in coronary surgery. Almost 2 decades ago, in a study that included more than 5900 patients, Loop et al35 showed that the use of the IMA on the LAD improved survival and reduced the incidence of new infarctions, new hospitalizations and reoperation at 10 years. Cameron et al36,37 confirmed that the benefits of this strategy persist after 15 years and 20 years.
These results made it possible to envisage that the additional use of the right IMA should improve the clinical results of CABG. However, verifying this intended benefit is proving difficult. The prognostic importance of LAD disease is higher than that of the other vessels and its revascularization with the left IMA ensures clinical benefit for more than a decade. To demonstrate the advantages of using the second IMA, prospective studies with a very long follow-up would be required in which the 2 revascularization strategies were compared in a large number of patients. These types of study have not been done up to the present, and therefore the information we have available is based on retrospective studies where patient selection, graft type (pedicled or free) and choice of the coronary artery to which the second IMA is targeted might have had a determining influence on the results.
In studies with a follow-up longer than 10 years, a significant decrease in the recurrence of new ischemic events is practically invariable when the second IMA has been used. Fiore et al38 found in a case-control study that using the second IMA for the RCA significantly decreases the recurrence of angina, myocardial infarction and the total incidence of ischemic events at 15 years. In a similar study, Pick et al39 found that when the second IMA is used for left coronary branches, the recurrence of angina at 10 years decreases to half (33% vs 63%). The incidence of new infarctions and the composite end point of any ischemic event were also lower in the patients receiving both IMA. Buxton et al,40 in a Cox proportional hazard model, have also confirmed that the presence of new infarctions and the need for reoperation are less at 12 years in patients in whom both IMA were used. In contrast, Sergeant et al41 concluded that, although the recurrence of angina is less in patients in whom both IMA are used, the difference is only 5% at 10 years. In our context, López Rodríguez et al42 have also identified the use of both IMA as an independent protective factor against the recurrence of angina, the need for new revascularization procedures and the composite end point of any cardiac event at 15 years. More recently, in a retrospective study of 4382 patients with multivessel disease, Stevens et al43 confirmed that the incidence of infarctions and new ischemic events is significantly less when both IMA are used.
Regarding survival, the benefits of using both IMA are less clear. In the greatest series published to date, and with a longer follow-up time, only Lytle et al44 found better survival rates at 10 years and 20 years in patients who received both IMA, and those who benefited more were the patients at greater risk. Pick et al39 report that mortality due to cardiac causes at 10 years is less in the patients receiving both IMA, although total survival is similar. Stevens et al43 also found that 10-year survival was 5% higher in the patients who received both IMA, although their clinical profile was also more favorable. In a metaanalysis carried out on more than 11 000 patients adjusted for age, sex, ventricular function, and the presence or otherwise of diabetes, Taggart et al45 confirmed the survival benefit of using both IMA. However, when the second IMA is targeted at the RCA or its branches, the survival benefits are less clear.44,46
Nevertheless, it should be taken into account that the clinical benefits of the use of both IMA have only been shown in patients where these arteries have mainly been used in situ, pedicled or targeted at branches of the left coronary artery. It remains to be confirmed whether other technical variations, such as the use of free grafts or the skeletonization of the IMA, achieve the same results.
Limitations and Drawbacks
The use of the IMA in situ is contraindicated in patients with stenosis of the brachiocephalic trunk or the ipsilateral subclavian artery, although in these cases it can still be used as a free graft. Neither can it be used when the IMA itself presents some intrinsic disease, as found in the patients with aortic coarctation or a history of thoracic radiation. A relative contraindication, especially when using both IMA, is the need to ensure immediate high coronary flow, as in patients with acute coronary syndrome (ACS), serious ventricular hypertrophy, and in reoperations where the IMA replaces a patent diseased vein grafted to the LAD, all of which are discussed later.
The drawbacks are related almost exclusively to the use of both IMA. This strategy increases intervention time and the morbidity related to the procedure, especially the incidence of sternal complications. Researchers at the Cleveland Clinic have demonstrated that the use of both IMA practically doubles the incidence of sternal dehiscence and/or mediastinitis, especially in diabetic patients.44,47 The use of both IMA has also been linked to a greater need for transfusions and reoperation due to bleeding and respiratory complications, which has led to this strategy being restricted to young patients without diabetes, obesity or chronic bronchopathy. Many of these relative contraindications have ceased to be so due to the increased experience of surgeons. However, it is undeniable that the mobilization of one, or especially, both IMA decreases sternal vascularity, especially when dissected as a pedicle.21 As mentioned, the skeletonization of one, or preferably, both IMA makes it possible to preserve sternal vascularity in most patients.
RADIAL ARTERY
In recent years, the RA has increased its popularity as an alternative arterial conduit due to the multiple advantages its use confers. It is less demanding to dissect than the IMA and gives rise to fewer complications. It is long enough to reach distal coronary branches and enables multiple sequential anastomoses. Due to its caliber and wall thickness, it is relatively manageable and is easier to anastomize to the aorta than the right IMA. Due to its technical versatility, many surgical teams currently consider it the second graft of choice after the IMA.48,49
Technical Options
The RA of the left forearm is normally used as this is, in general, the non-dominant one, since its extraction does not interfere with that of the left IMA. However, the RA can be extracted from the right forearm or even both can be used. The RA is, by definition, used as a free graft and is anastomized proximal to the aorta or to another graft.
The most accepted indication to use the RA is revascularization of the second most important coronary artery in patients in whom the use of both IMA is contraindicated. Thus, this conduit is normally targeted at the branches of the LCX or the RCA. Less frequently, the RA is used on diagonal branches and in cases where neither of the IMA can be used, the RA is used to revascularize the LAD. When the RA is long enough it can be divided into 2 segments to obtain independent grafts,50 or it can be used as a sequential graft to carry out multiple distal anastomoses.51
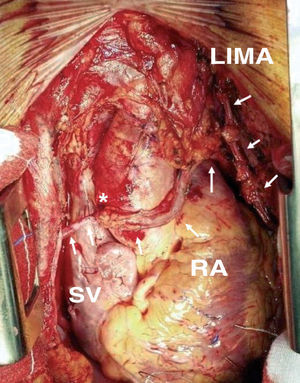
The best location for the proximal anastomosis of the RA is a matter of dispute. Many surgeons prefer to graft it directly to the ascending aorta.52 However, the different thickness and caliber of both vessels and the frequent presence of atheromatous plaque in the aorta can jeopardize the patency of this anastomosis. To eliminate these drawbacks anastomosis to the origin of a SV graft (Figure 4) or to a pericardial patch previously sutured to the aorta is recommended.53 In contrast, other groups defend the use of the inverted Y or T proximal anastomosis to an IMA graft54 (Figure 5), which makes it possible to reach more distant coronary vessels and decreases the need to handle the aorta.
Figure 4. Radial artery graft anastomized proximal to the origin of a saphenous vein graft targeted at the right coronary artery. LIMA indicates left internal mammary artery; RA, radial artery; SV, saphenous vein. *End-to-side anastomosis between the left internal mammary artery and the radial artery.
Figure 5. Composite T-graft with in situ pedicled left mammary artery to the anterior descending artery, and skeletonized radial artery to a diagonal branch and the obtuse marginal artery. LIMA indicates left internal mammary artery; RA, radial artery; DB, diagonal branch; OM, obtuse marginal artery.
Patency
Generally, the short-term patency of the RA is excellent; most studies establishing it between 90% and 95% after 1 or 2 years.26,48,55-57 Nevertheless, this information should be interpreted with caution, since most of the studies are retrospective and only include a small proportion of patients in whom this type of graft was used. As in other types of conduits, the patency of the RA is higher when prospectively studied than when studied due to the reappearance of symptoms. In a prospective study currently in progress, the total patency of the RA at 1 year was 93%, while that of symptomatic patients was 86%.27 In the RAPS (Radial Artery Patency Study), the RA was used as an alternative to the SV and was randomly assigned its target artery (LCX or RCA) to avoid the influence of the type of target coronary artery.58 The percentage of grafts occluded after 1 year was significantly higher in the SV (13.6% vs 8.2%). However, when analyzing the joint incidence of occluded grafts or with a diffuse decrease in caliber, the percentage of non-functional conduits was similar with both grafts (15.2% vs 14.4%). In addition, more than 20% of the patients with patent RA grafts presented some degree of stenosis in the proximal anastomosis. To date, only the mid-term term results of the RAPCO (Radial Artery Patency and Clinical Outcomes)59 study have been published out of the various prospective and randomized studies. In this study, in addition to the left IMA to the LAD, the patients received the right IMA in the second most important vessel if they were <70 years old or the RA if older. In the latter group, the difference in patency between the RA and SV at 5 years did not reach statistical significance (87% and 94%, respectively). In some observational studies, an even higher rate of occlusion and/or stenosis has been found in the mid-term in the RA than in the SV.60 In contrast, in another observational study in the longer term, RA patency at 9 years was 88% which, although less than the 96% when using the IMA, was significantly higher than the 53% with SV grafts in the same patients.61
In retrospective studies comparing patency in the RA and right IMA, very variable angiographic results have been found, with patency oscillating between 53% and 95% with the RA and 79% and 100% with the right IMA.28,60 In the patients <70 years old included in the RAPCO study, mid-term patency in the right IMA was similar to that of the RA (95% and 100%, respectively).59
The early occlusion of the RA is generally due to technical causes whereas localized stenoses are caused by injury to the artery during dissection. Spasm, which appears in the first days or weeks, is local, and is resolved with systemic or intra-arterial vasodilators. However, the most frequent mechanism of RA graft failure is a diffuse decrease in its caliber, an event known as "string sign" due to its angiographic appearance (Figure 6). This event especially happens in circumstances of low blood flow through the graft, as occurs when there is a dominant flow in the native coronary artery or when revascularizing poor run-off coronary arteries. This occurs as an adaptation of the RA to such hemodynamic conditions.28 The natural evolution of grafts with this angiographic anomaly remains unknown. Although it has been suggested that they can recover normal angiographic appearance once stenosis in the coronary artery has evolved, there is no evidence to assert this will be so in all cases.
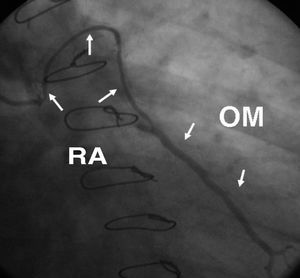
Figure 6. Angiographic appearance (string sign) at 6 months following radial artery graft surgery targeted at a large caliber marginal branch with noncritical stenosis. RA indicates radial artery; OM, obtuse marginal artery.
Both the degree of stenosis in the coronary artery and the flow demand of the revascularized territory are factors that independently effect the patency of RA grafts. Radial artery patency is excellent when targeted at occluded arteries or those with serious stenosis, whereas it decreases significantly when stenosis is <80%.62 Other authors have found that if coronary stenosis is <90%, the risk of occluding the RA is 3 times greater than when this is not the case.58 In the RAPS study, early occlusion of the RA was 5.9% in arteries with <90% stenosis and 11.8% when this was 70%-89%.60 On the other hand, in the territory of the LAD, RA patency is very similar to that obtained with the IMA, decreasing with the LCX and, especially, when the RA is targeted at the RCA or its terminal bra nches.28,62,63 Thus, it would be advisable to reserve the RA for use in arteries with a high degree of stenosis and in which high run-off is predicted.
Although attention has been drawn to other factors determining RA patency, their influence has not been clearly demonstrated. The different ways of carrying out proximal anastomosis, either to the aorta or some other graft, do not seem to influence RA patency.56,64,65 Neither the caliber of the target coronary artery nor the quality of its wall seem to affect RA patency.63 On the other hand, neither has it been demonstrated that prolonged use of calcium antagonist therapy improves long-term patency in the RA.61,63
Clinical Results
The clinical results of myocardial revascularization using the RA as a second arterial graft are comparable to and even better than those obtained with IMA and SV grafts. In some of the published series with a higher number of patients, the use of the RA is not only not associated with increased early morbidity and mortality, but is even less than that with standard intervention.48,66 There are some studies in which the use of the RA is associated with less perioperative morbidity and mortality and a lower incidence of complications deriving from harvesting the graft.49,66
Regarding mid-and long-term results, different observational studies have reported very favorable results with the use of the RA.48,67,68 When the clinical evolution of patients in whom the RA has been used as a second graft is compared to those in whom this has been done with the right IMA, no significant differences have been found, with a very low incidence of recurring ischemic events under both strategies.69,70 In the RAPCO study,59 the percentage of patients free of ischemic complications at 4 years was similar when the use of the RA (84%) was compared with the SV (89%) or when comparing the RA (91%) and the right IMA (82%). On the other hand, in a prospective observational study, Caputo et al49 found that survival free of ischemic events at 18 months was significantly better when the RA was used as a second arterial graft (98%) than when done with the right IMA (92%), despite the patients in the first group being at higher risk. In Spain, Dalmau et al71 found identical results in a similar study.
Limitations and Drawbacks
As already mentioned, the main limitation to using the RA is the coronary anatomy; the receiving artery should have severe stenosis, >80%, and irrigate a territory in which high flow debt is anticipated. This excludes arteries with diffuse disease or irrigating small territories or those having little viable myocardium. Furthermore, the RA cannot be used when the ulnar artery by itself cannot irrigate the hand sufficiently. This possibility should be ruled out in all patients before the intervention via the Allen test or one of its variations.
Another contraindication is serious calcification of the artery wall, being more frequent in older or diabetic patients. Neither can the RA be used in patients with Raynaud disease, Dupuytren disease, or scleroderma, or in those who have a history of injury to or prior surgery in the arm, or with a known disease in the subclavian and humeral arteries. Its use is also contraindicated in patients with serious kidney failure due to its possible use in arteriovenous fistula.
The main drawback is the tendency of the RA to spasm, which requires a preventative pharmacological strategy, especially during the first weeks. In different studies the administration of certain pharmacological agents has proven advantageous, either topically during graft preparation, intravenously during the hours following surgery or oral once eating is resumed. During graft preparation papaverine, phenoxybenzamine, and mixtures of verapamil and nitroglycerin in solution have been used. Some researchers recommend nitroglycerin due to its greater efficacy against spasm, fewer side effects, and low cost.72,73 However, Mussa et al74 have recently discovered that phenoxybenzamine has a longer protective effect than nitroglycerin and preserves endothelial function better than papaverine.
Most authors recommend introducing calcium antagonists once eating is reestablished and maintaining them over a variable period, never <6 months.28,61,68 Among the maintenance treatment drugs, the dihydropyridines and nitrates seem to be more effective than diltiazem and verapamil for preventing and treating spasm.75 Nevertheless, to date, the type and dose of the ideal drug has not been demonstrated, nor the length of administration time, nor even whether this therapy is essential. In a recent study, Gaudino et al76 studied the incidence of ischemic events, myocardial perfusion quantified via gamma radiography with radioactive isotopes, angiographic appearance, and the vasomotor response in vivo to the administration of serotonin. At 1 year no advantage was conferred with diltiazem at the normally recommended dose of 120 mg.
Manifestations of arterial insufficiency after RA harvesting are rare providing suitable preoperative assessment has been done. Neurological changes, mainly sensory, are slightly more frequent, although rarely incapacitating. Their incidence is always <10% and in most cases completely disappear in a few days or weeks.77 Infection of the surgical wound and hematomas are rare.51,52
GASTROEPIPLOIC ARTERY
Technical Options
The GEA is normally used as an in situ graft to revascularize the lower part of the heart. In general, it is targeted at the distal RCA, PDA or, less frequently, at the marginal branches of the LCX or even to the distal LAD. In most patients this is done in conjunction with the left IMA or RA, in an attempt to obtain complete revascularization with arterial grafts. However, it can be used in isolation in the few patients who only require revascularization of the RCA or its distal branches. This normally presents more frequently in patients already having undergone intervention with a patent IMA graft to the anterior side of the heart. In these circumstances the RCA can be revascularized via a small incision in the epigastrium, so avoiding the risk of a new sternotomy and the manipulation of the patent venous grafts.78
The use of the GEA as a free graft is less common; the end with the greatest caliber is anastomized to the ascending aorta or, as a component of a composite arterial graft, anastomized proximal to an IMA or RA graft. Whenever the caliber of the GEA allows, more than one artery can be revascularized with it via one or more side-to-side anastomoses.
Patency
The short-term patency of the GEA is comparable to that of the IMA. In the long term, the patency rate is lower than that of the latter being slightly higher than 80% at 5 years and 60% after 10 years.79,80 In a study by Sum et al,80 GEA patency at 10 years (63%) was significantly less than that of the IMA (94%) but very similar to that of the SV (68%). More recently, Hirose et al81 studied the angiographic results of 1000 patients with GEA grafts targeted particularly at the RCA, finding that GEA and left IMA patency was 98% and 99% at 1 year and 84% and 97% at 3 years, respectively. Nevertheless, it should be taken into account that most angiographic studies were done in patients with recurrent angina, which could underestimate actual patency and that, normally, the GEA is used to revascularize RCA branches, a territory in which any type of graft has less patency in the mid- to long term. As occurs in the RA, the main failure mechanism in the GEA is the diffuse decrease in the graft lumen or its occlusion when used to revascularize mildly stenotic arteries or those with poor run-off.
Clinical Results
In the hands of experienced surgeons, the use of the GEA does not increase perioperative morbidity and mortality,82,83 and neither have complications been reported arising from partial deprivation of arterial flow to the stomach. The long-term clinical results of using the GEA (normally associated with one or more arterial grafts) are excellent, with a survival free of new ischemic events >80% at 10 years.79,82
Limitations and Drawbacks
The need to enlarge the incision to access the abdominal cavity is less of a drawback once the surgeon has become familiar with the technique. In contrast, an earlier abdominal intervention is a drawback and this type of graft would be problematic if a future laparotomy is required. Other possible limitations arise from the difficulty in ruling out possible stenosis in the celiac trunk, which is particularly frequent in patients with serious peripheral vascular disease, and the problems involved in carrying out postoperative angiographic control.
OTHER ARTERIAL GRAFTS
Other arteries are much less frequently used in myocardial revascularization. They are normally resorted to when, due to some exceptional circumstance, other conduits are not available to achieve complete revascularization with arterial grafts. The inferior epigastric artery is the one most commonly used. It is normally used as a free graft and targeted at vessels of the anterior side of the heart. It can also be used to extend another arterial graft, generally the IMA or, less frequently, the RA. Buche et al84 found an early patency of 86% when anastomizing it to the aorta, a technique not without difficulty, whereas Calafiore et al85 reported a patency of 93% at 12 months when anastomizing it to another arterial graft. However, variability in the length of usable artery, which is often insufficient to reach distal arteries, plus the difficulty of the proximal anastomosis have prevented its use becoming more general.
COMPOSITE ARTERIAL GRAFTS
During the last decade, many technical variations have been developed for so-called complex arterial revascularization, consisting of linking different types of arterial grafts with a double aim: a) to extend the length of IMA or RA grafts to reach territories not reached by the in situ IMA or RA anastomized to the aorta, and thus to revascularize the greatest possible number of coronary vessels with arterial conduits; and b) to totally avoid the manipulation of the ascending aorta in patients with suspicion or evidence of serious atheromatosis, with the aim of diminishing the risk of perioperative neurological complications.
Technical Options
The technical variant most used is anastomizing the free right IMA or RA to the body of the in situ left IMA as an inverted Y- or T-graft so as to revascularize all the branches of the left coronary artery. This strategy, combined with the use of the right IMA or GEA on the RCA, makes it possible to obtain complete revascularization with arterial conduits in many patients with multivessel disease. The other way to construct a composite arterial graft is to extend one of the IMA with the other or with another type of arterial conduit, normally the RA, and to carry out multiple side-to-side anastomoses with this long graft. Nevertheless, multiple technical variations have been described in which both the type of artery used and the connection method have been modified, either by extending one to the other or laterally anastomizing them as inverted Y, T, U, or K grafts. For example, extensive arterial revascularization can be achieved in the inferior-posterior and lateral sides of the heart via a composite GEA and RA U-graft; the distal end of the GEA is anastomized end-to-side to the body of the RA, using this to revascularize whatever branches of the RCA and LCX that require it.86
Patency
In line with the results of one of the more experienced teams, when an IMA is used as a composite graft, long-term patency is similar to that of in situ grafts.87 It has even been suggested that the RA could function better when anastomized proximally to the IMA than to the aorta (Buxton BF, personal communication). On the other hand, other authors have found that the early patency of the right IMA is lower than when used in situ.26,88 In a randomized study in which the patency of the RA and GEA were compared when anastomized to the IMA as a Y-graft and targeted at revascularizing branches of the left coronary artery, Santos et al89 found that early patency was significantly better in the RA (89.6%) than in the GEA (68.9%).
Clinical Results
In the light of the apparent advantages of revascularization with the 2 IMA, it would seem natural to assume that exclusive revascularization with arterial grafts should optimize the clinical results of CABG. Combining both IMA with the GEA or RA, either as independent or composite grafts, makes it possible to achieve this aim in a high proportion of patients. Using the 2 IMA and the GEA, Bergsma et al90 found an actuarial survival rate of 91% at 7 years, with 85% of the patients free of angina. The results of 2 more observational studies with long-term follow-up have recently been published. In both studies, a very high percentage of patients (69% and 77%, respectively) were alive and without signs of myocardial ischemia at 10 years, and no patient needed a new intervention.91,92
Despite these excellent clinical results, this strategy should be approached with caution since, generally, it has been used in selected patients plus the fact that there are no rigorous prospective studies showing its benefits in the general population.
Limitations/Drawbacks
The preparation of composite arterial grafts is technically demanding, often increasing intervention time, and is associated with greater early morbidity.93 However, once the learning curve is completed, it can be done with the same level of risk as conventional revascularization.
The main concern regarding this revascularization strategy is the capacity of the left IMA, on which the perfusion of the entire left coronary system depends, to take up the flow demand, especially in circumstances where it is predicted as being particularly high. The intraoperatorive evaluation of composite graft branch flow via ultrasonic devices has demonstrated values comparable to those of individual conduits in the absence of technical errors.94 Affleck et al95 evaluated free flow in IMA and RA composite grafts and at the conclusion of CPB once they were anastomized to the coronary bed, and argued that flow reserve was almost double in the final measurement. However, in a more clinical context, Sakaguchi et al96 compared myocardial perfusion quantified via positron emission tomography in 2 groups of patients revascularized with independent arterial grafts or composite arterial Y-grafts, both at rest and after dipyridamole infusion. The flow reserve assessed in four ventricular areas was significantly lower in all of them in patients revascularized with composite grafts, suggesting that this technical variant has less capacity to supply the myocardium under conditions of stress.
USE OF ARTERIAL GRAFTS IN SPECIAL CIRCUMSTANCES
Diabetic Patients
Coronary disease in diabetic patients is often diffuse, involving multivessel disease, multiple lesions and poor quality distal beds, especially in insulin-dependent patients. In this anatomical context, long-term patency in SV grafts is especially compromised. The use of the IMA in diabetic patients has led to a better functional situation and less angina recurrence, especially in the subgroup of patients with depressed ventricular function.97 The clinical benefit of revascularizing the LAD with the IMA has been demonstrated in prospective and retrospective studies with long follow-up, where the survival of diabetic patients was similar to that of non-diabetic patients.98,99 The extensive use of arterial grafts has been viewed as a way to improve the results of conventional surgery. In line with this, Endo et al100 have demonstrated that the use of both IMA significantly reduces the need for new interventions and the incidence of new infarctions in diabetic patients, although it does not increase survival. In brief, at present we still do not have information that unquestionably demonstrates that the use of both IMA or supplementing the left IMA with any other type of arterial graft or even complete revascularization with arterial grafts can counteract the damaging effect of diabetes in the mid- and long term.
However, on the other hand, diabetic patients often present intrinsic arterial disease that limits the number of conduits available. Furthermore, diabetes is a factor that has a clear relationship with complications due to sternotomy, especially when the two IMA are used. Various studies have demonstrated that skeletonization makes it possible to use both IMA in diabetic patients with a risk of sternal complications very simil ar to that in non-diabetic patients.101-103 However, alternative strategies have to be followed to use both IMA in diabetic patients where several risk factors for sternal dehiscence combine, such as obesity and chronic obstructive pulmonary disease. Matsa et al102 reported an incidence of sternal complications of up to 15% in obese diabetic women.
Elderly People
There is an ever-increasing population of older people recommended for coronary surgery. It has been argued for many years that the limited life-expectancy of elderly people does not justify the use of arterial grafts due to the greater morbidity that can occur in this group of patients. On the other hand, in older people, the SV is often of poor quality and the aorta presents serious atheromatosis; only the use of arterial conduits can overcome these problems. In the last decade, Gardner et al104 and Azariades et al105 already demonstrated that the systematic use of an IMA in patients >70 years old was not associated with a greater incidence of early complications. In fact, this strategy reduced hospital mortality and improved 5-year survival by 10%. According to the experience of Galbut et al,106 a still greater benefit can be obtained, at the same level of operative risk, when both IMA are used. Nevertheless, when assessing the results of these retrospective studies the possibility of bias regarding candidate selection for one technique or another cannot be discarded. Recently, in a prospective study carried out with patients >70 years old, Muneretto et al107 confirmed that revascularization done exclusively with arterial grafts does not lead to greater morbidity and mortality, and provides better angiographic and clinical results than conventional surgery, even in the mid-term.
Left Main Coronary Artery Disease
The possibility of arterial grafts, whether single or multiple, not being able to provide sufficient coronary perfusion has been one of the fears limiting their use in patients with severe stenosis of the left main coronary artery (LMCA). Barner et al108 demonstrated that using both IMA to revascularize both branches of the LMCA is a safe technique which does not involve greater morbidity and mortality than using SV grafts. On the other hand, when analyzing the long-term clinical results, Galbut et al109 found a 5-year survival rate of 82% in 276 patients with LMCA disease revascularized with both IMA. Furthermore, the exercise stress test yielded a negative result in most patients (92%). These data, when combined with that demonstrating the capacity of the IMA to increase its caliber in response to flow demand, bears out the ability of the 2 IMA to meet left coronary circulation demand.
Reinterventions
Patients requiring reintervention often present diffuse coronary disease, a situation where mid- and long-term venous graft patency is poor. Such patients can benefit from the use of one or both IMA as well as any arterial grafts considered appropriate which have not been used in the previous intervention, without involving added risk. In the Cleveland Clinic group's experience, the use of at least one IMA in this group of patients significantly improved 10-year survival.110 Both IMA can be used in reinterventions, although this involves a higher incidence of myocardial and respiratory complications.111 Although technically feasible, this strategy should be avoided in obese and diabetic patients or in those who have severely depressed ventricular function.
On the other hand, disease progression in native coronary arteries in patients who need a second intervention means that most of the myocardium depends on the grafts done in the first one. Some of them can be patent but sufficiently diseased to justify their substitution at the time of reintervention. The substitution of functional venous grafts for an arterial graft can immediately compromise myocardial perfusion. When a diseased venous graft is replaced, on which a large amount of myocardium depends, the incidence of hypoperfusion is high, up to 19%, as well as the operative mortality caused by this complication.112 To prevent this complication without abandoning the advantages provided by the IMA, the Cleveland Clinic group recommends anastomizing the IMA distally to the venous graft without occluding it, while accepting a small risk of atheroembolism due to manipulating the diseased SV.113,114
Emergency Procedures
Another special situation is the need to perform an emergency revascularization in the context of ACS or when complications have arisen due to coronary intervention. In these circumstances specific facts combine to possibly counterindicate the use of arterial grafts, or at least their most complex variants. On the one hand, revascularization needs to be done urgently, entailing rapid harvesting of conduits, which is more demanding in the case of arteries. On the other hand, in this situation, it is usually vital to ensure immediate and sufficient coronary perfusion, which arterial conduits cannot always guarantee. For these reasons many surgeons prefer not to use arterial grafts, especially for revascularizing the ACS culprit lesion. In contrast, others defend the use of the IMA, preparing it once CPB has been started or even after having stopped the heart while under retrograde cardioplegia. Among these, Zapolansky et al114 have used the left or right IMA to revascularize the ACS culprit lesion without finding a greater incidence of perioperative ischemic complications. However, in the experience of other authors, this practice entails supplementing the IMA with an SV graft to the same vessel in up to 40% of patients.115 In this context, it seems reasonable to restrict the use of arterial grafts, and especially their extensive use, to patients in a stable hemodynamic condition, without progressive ischemia and who do not present other contraindications.
FUTURE PROSPECTS
Without question, the future of CABG involves greater use of arterial conduits in the attempt to increase the duration of clinical benefits. However, the challenge from interventionist cardiology is also motivating surgeons to seek less aggressive methods to carry out revascularization.
The manner in which conduits are obtained is basic to their optimal working. The use of endoscopic techniques and new instruments, such as the harmonic scalpel, can minimize tissue damage and reduce the incidence of wound infections as well as neurological injury.116 Reducing ischemia time in conduits and using drugs that make it possible to better preserve their physiology are also aspects that can prevent early dysfunction and histological changes in the mid- and long-tem.117
Minimizing CPB circuits and beating heart revascularization are ways to reduce the type of aggressive surgery used for years, but whose indications have still not been totally clarified. Minimally invasive, video-assisted or even robotic surgery, as well as anastomosis done via automatic connectors, are all being explored and may have a role to play if the clinical results support this. All these strategies will be thoroughly discussed in other chapters in this series.
Section Sponsored by the Dr Esteve Laboratory
Correspondence: Dr. J.M. González Santos.
Servicio de Cirugía Cardíaca. Hospital Universitario de Salamanca.
P.o San Vicente, s/n. 37007 Salamanca. España.
E-mail: jmgs@usal.es