Keywords
INTRODUCTION
All percutaneous revascularization techniques rupture atherosclerotic plaque and denude the vascular endothelium to a greater or lesser extent, leading to this material and subendothelial tissue being exposed to the bloodstream. This stimulates adhesion and platelet aggregation as well as the coagulation cascade. Both processes lead to thrombin production (thrombus formation) and ultimately to fibrin production (for thrombus stabilization), which might have repercussions on the coronary lumen. Thus, since the beginning of percutaneous coronary intervention (PCI), multiple strategies have been tried to reduce the acute complications stemming from thrombus formation, mainly based on the administration of drugs with antiaggregation and anticoagulant activity.
Initially, angiographic flow and residual stenosis in the coronary lumen were taken as parameters to evaluate the success of the intervention. However, it was soon observed that, independently of epicardial coronary flow, optimal reperfusion, with prognostic implications, also guaranteed myocardial and microvascular reperfusion. Adequate myocardial perfusion is hindered by microvascular dysfunction mainly due to the ischemia itself, reperfusion injury, and distal embolization of thrombotic and atherosclerotic material during the procedure. Different strategies have been tried to minimize these events occurring during PCI. Regardless of the distal protection devices, the drugs used for this purpose mainly relate to the treatment of acute ST-segment elevation myocardial infarction. As this topic deserves its own space they will not be described in detail here.
Finally, a third problem of concern to the catheterization specialist is restenosis of the coronary artery in the mid- and long-term. In addition to strict control of cardiovascular risk factors and secondary prevention drugs in ischemic heart disease, drug-eluting stents are presented as the main alternative to reduce the incidence of restenosis. From the pharmacological standpoint, promising outcomes have recently been achieved with certain immunosuppressive drugs, the process of restenosis being understood as a general inflammation process.
In the following, the main adjuvant drugs for PCI are presented. First, drugs with antithrombotic action, which are basic to PCI, are described in detail; second, drugs that have recently proven to reduce the incidence of coronary restenosis are briefly described, this being the case of oral sirolimus.
ANTITHROMBOTIC DRUGS (I): ANTIPLATELET AGENTS
Acetylsalicylic Acid
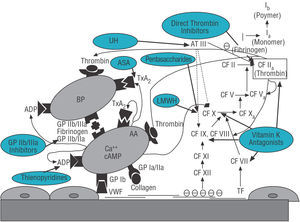
Aspirin or acetylsalicylic acid (ASA) acts by irreversibly inhibiting platelet cyclo-oxygenase-1 that synthesizes thromboxane A2 (TxA2) from arachidonic acid. The inhibition of TxA2 synthesis, one of the main potentiators of platelet aggregation, gives rise to the antiplatelet effect of ASA (Figure 1).
Figure 1. Simplified diagram of the primary and secondary hemostasis systems and effects of the main antithrombotic drugs. AA indicates arachidonic acid; ASA, acetylsalicylic acid; AT III, antithrombin III; CF, coagulation factors; VWF, von Willebrand factor; GP, glycoprotein platelet receptors; LMWH, low-molecular-weight heparins; UH, unfractionated heparin; BP, blood platelet; TxA2, thromboxane A2.
The studies that established the benefit of ASA therapy in the context of PCI date to the end of the 1980s. Compared to placebo, they demonstrated a reduction in acute ischemic events, such as periprocedural thrombosis and acute myocardial infarction (AMI).1-3 Indefinite ASA therapy continues to be the indisputable indication in patients with ischemic heart disease referred for cardiac catheterization, and its usefulness in secondary prevention (reduction in death rates, reinfarction, or stroke) has been fully demonstrated.
On the other hand, the antiinflammatory role of low-dose (80 mg/day) ASA has been recently described. This action, apart from its antiplatelet effect, helps to partly explain the benefits attributed to the drug in cases of ischemic heart disease and specifically in PCI. In general, it is accepted that a dose of 80-325 mg/day ASA should be administered at least in the 2 h prior to the procedure.4,5 However, these doses are empirical, without an effective minimum dose having been definitively established. When ASA therapy is combined with other antithrombotic drugs (mainly clopidogrel or acenocoumarol), yet lower doses (75-100 mg/day) are recommended based on a substudy of the CURE study, which demonstrated a higher incidence of major hemorrhages in the groups that received doses of ASA higher than 100 mg/day (maximum incidence in the ASA group receiving >200 mg/day).6
Thienopyridines
The thienopyridines (ticlopidine and clopidogrel) act by irreversibly inhibiting the adenosine diphosphate platelet receptor (Figure 1), and thus its antiplatelet effect complements that of ASA. This means that, since their introduction, these drugs have been mainly studied in combination with ASA, in an attempt to obtain a stronger antiplatelet effect, since, with the introduction of the intracoronary stent, the subacute thrombosis rate with single antiplatelet therapy with ASA still reached 3.5%-8.6%.
Like ASA, in addition to their antiplatelet effect, the thienopyridines, specifically clopidogrel, have an antiinflammatory effect because they eliminate certain inflammation markers such as CD62 and CD40L and reduce the increase in C-reactive protein that follows coronary catheterization, which has prognostic value.
Ticlopidine
In several studies ticlopidine combined with ASA has demonstrated its superiority versus ASA only or ASA plus warfarin. In the STARS study, 1653 patients with "relatively low thrombotic risk" were randomized to treatment with ASA only (325 mg/day), ASA (325 mg/day) plus ticlopidine (500 mg/day), or ASA (325 mg/day) plus warfarin. The primary 30-day endpoint (the combination of death, culprit vessel revascularization, AMI or subacute thrombosis) was only 3.6% in the ASA-only group, 2.7% in the ASA plus warfarin group, and 0.5% in the ASA plus ticlopidine group (P<.001).7 Similar results were obtained in the ISAR and MATTIS studies that evaluated the benefit of ticlopidine in patients with greater thrombotic risk.8,9
The frequent, and at times severe, side effects of ticlopidine have currently relegated it to anecdotal cases. Gastrointestinal effects (20%) have been described as well as cutaneous reactions (4.8%-15%) and changes in liver tests, although, without doubt, its most feared unwanted effects are serious neutropenia (1% of cases, usually reversible) and thrombocytopenic thrombotic purpura (<1/1000 and usually fatal).
Clopidogrel
Clopidogrel is a thienopyridine-family drug that was introduced as an alternative to ticlopidine in an attempt to reduce side effects while maintaining similar therapeutic efficacy. The CLASSICS study was one of the first with this aim. It included more than 1000 patients undergoing catheterization and ASA therapy, randomized to ticlopidine (500 mg/day), clopidogrel (75 mg/day), or clopidogrel with loading doses (300 mg in bolus followed by 75 mg/day). It demonstrated a reduction in the primary endpoint (the combination of major hemorrhage, neutropenia, thrombocytopenia, or discontinuation of treatment) in the clopidogrel treatment groups (4.6% for both groups together vs 9.1% for ticlopidine group; P<.005).10 There were no differences in death rate, AMI, or need for revascularization. Like the CLASSICS study, other studies have confirmed the lower incidence of unwanted side effects with clopidogrel with no difference in efficacy between the agents.11 However, in a metaanalysis that included almost 14 000 patients, there was a reduction in mortality (0.48% vs 1.09%; P=.003) and in the group of major ischemic events (2.1% vs 4%; P=.002) with clopidogrel therapy versus ticlopidine therapy,12 which definitively established clopidogrel as the better treatment.
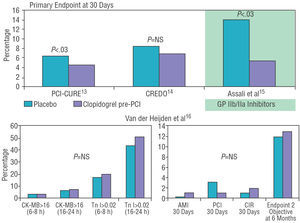
Two recent studies established the benefit of clopidogrel when administered prior to PCI (Figure 2). In the PCI-CURE study, the administration of clopidogrel over an average of 10 days before PCI in the patients with acute coronary syndrome (ACS) was related to a reduction in major periprocedural events (death, AMI or emergency revascularization) and at 30 days (4.5% vs 6.4% in the placebo group; P=.03).13 On the other hand, the CREDO study showed a reduction (non-significant) of 38.6% in the primary endpoint after administering clopidogrel a minimum of 6 h before elective catheterization compared to the group that did not receive pretreatment.14,15 Such benefit was especially marked in the patients who received earlier pretreatment (>15 h before catheterization). Recent data on stable ACS establish that, compared to placebo, pretreatment with clopidogrel does not reduce intraprocedural myocardial damage as determined by elevated necrosis markers (creatine kinase isoenzyme MB and troponin I), nor improve the clinical picture of the patients at 30 days and 6 months.16 As a result, indications for treatment with clopidogrel prior to cardiac catheterization are still not established.
Figure 2. Results of studies evaluating the benefit of clopidogrel treatment prior to percutaneous intervention. RVS indicates revascularization surgery; CK-MB, creatine kinase MB isoenzyme; AMI, acute myocardial infarction; GP IIb/IIIa, GP IIb/IIIa inhibitors; PCI, percutaneous coronary intervention; NS, not significant; objective 2, secondary objective; Tn, troponin.
In general, a 300 mg loading dose of clopidogrel is recommended, ideally more than 6 h before catheterization. If catheterization is anticipated before 6 h, a larger loading dose (600 mg) can be administered, although this hypothesis should be confirmed with randomized studies.5 Similarly, initiating ticlopidine treatment a minimum of 72 h beforehand is recommended to ensure a good level of antiplatelet action at the time of the procedure. These strategies, however, are not globally accepted in clinical practice, in view of the fact that pretreatment with double antiplatelet agents, in addition to their limited benefit, can be counterproductive in certain circumstances, as in the case of the need for emergency revascularization surgery or the appearance of some complication during PCI (coronary perforation or dissection). Thus, the administration of the loading dose after stent implantation continues to be the current preference of many catheterization specialists.
After PCI with stenting, the classic treatment is double antiplatelet therapy (ASA plus thienopyridine) for a month, which is the agreed period during which the majority of thrombotic complications deriving from the procedure occur.17 Recent studies have demonstrated the long-term benefit of clopidogrel therapy after PCI. The PCI-CURE study obtained better results regarding combined death, reinfarction or need for revascularization in the group that received 9-month treatment with clopidogrel versus the group that received it for only 1 month as PCI aftercare (18.3% vs 21.7%; P=.03).13 Furthermore, prolonged treatment with clopidogrel was not associated with an increase in major hemorrhages, but was so with smaller ones (3.5% vs 2.1%; P=.03).13 On the other hand, the CREDO study prolonged the administration of clopidogrel to 12 months in patients undergoing elective catheterization, demonstrating a 26.9% reduction in major events (death, AMI, stroke) at 1 year compared to the patients who received post-catheterization clopidogrel for 1 month only (P=.02).14 However, it should be mentioned that the group of patients who received clopidogrel therapy for 1 month did not receive a loading dose, unlike the prolonged treatment group, and this could have biased the results. On the basis of these results, continuing treatment with double antiplatelet therapy (ASA plus clopidogrel) for 9-12 months after PCI is usually recommended,18 although some authors have a more conservative attitude alleging that prolonged therapy is not cost-effective.19 It has been recently shown that the implantation of drug-eluting stents that work by inhibiting endothelialization at the stent level produces, however, a persistent denudation of the vascular wall that can lengthen an inflammatory and thrombotic response that usually takes place in the first postprocedural month. Therefore, in the case of drug-eluting stents, double antiplatelet therapy is indispensable for a period longer than 1 month. Double antiplatelet therapy lasting 2 months and 3 months has been tested in the case of sirolimus-eluting stents, and 6-month therapy with paclitaxel-eluting stents. These have shown a 70-80% reduction in major events compared to conventional stenting while using the same antiplatelet therapy.20,21
Finally, it should be recalled that, in addition to their adjuvant role in relation to ASA in PCI, thienopyridines are the treatment of choice in the patients who are allergic to ASA or cannot tolerate it. Antiplatelet therapy should be started with these agents, preferably with clopidogrel, ideally 72 h before the procedure, if this is elective, and afterwards continued indefinitely.22
Glycoprotein IIb/IIIa Inhibitors
Glycoprotein (GP) IIb/IIIa inhibitors act, as their name indicates, by inhibiting the GP IIb/IIIa platelet receptors responsible for platelet aggregation through fibrinogen bridges (Figure 1). Unlike ASA or thienopyridines, GP IIb/IIIa inhibitors intervene in the final step in all pathways causing platelet aggregation, thus achieving a stronger antiplatelet effect.
To date, three main drugs have been tested in the context of ischemic heart disease (i.e., abciximab, eptifibatide, and tirofiban). In general, these three drugs have mainly shown a reduction in AMI rates, the need for emergency revascularization, or an improvement in angiographic flow obtained acutely, without attributing a direct role to them in the inhibition of restenosis in the medium- and long-term.5 Although no significant benefits have been obtained regarding mortality in any of the main studies, a recent metaanalysis, which included 12 studies with more than 20 000 patients, established a reduction in 30-day mortality in the patients treated with GP IIb/IIIa inhibitors versus placebo (0.9% vs 1.3%; odds ratio [OR]=0.73; P=.024), with an estimated 2.8 lives saved at 30 days per every 1000 patients treated.23
The 3 main drugs are described in the following, together with the results demonstrated to date in native coronary arteries.
Abciximab
Of the 3, abciximab (ReoPro®) is the drug that has definitely demonstrated its efficacy and the only one that has provided benefits in all contexts of patients with ischemic heart disease; namely, acute ST-segment elevation myocardial infarction,24,25 high-risk non-ST segment elevation ACS,25 low-risk ACS,26 and stable patients undergoing elective catheterization, even when no revascularization has been planned in advance.27
Abciximab is a chimerical monoclonal antibody (mouse-human) that selectively inhibits the GP IIb/IIIa receptor, causing prolonged platelet aggregation blockage, especially when administered as an infusion (up to 50% platelet aggregation inhibition 24 h after suspending infusion). Its immunological origin explains why possible hypersensitivity reactions with repeated administration were attributed to it. This was not confirmed in a registry of 500 patients undergoing repeated abciximab administration, where no case of anaphylaxis or other allergic manifestations was observed.28 The presence of human antichimeric antibody (HACA) IgG antibodies, that occurs approximately in 6% of the patients, was not related to any complication nor decreased the effectiveness of the drug. However, an increase in severe thrombocytopenia rates was found, and thus hematological monitoring of patients with HACA is currently recommended.
The EPIC study25 was the first to demonstrate the efficacy of abciximab. It included more than 2000 high-risk patients randomized to ASA plus heparin at fixed doses (10 000-12 000 U), to ASA plus fixed-dose heparin plus abciximab bolus (0.25 mg/kg) or to ASA plus fixed-dose heparin plus bolus (0.25 mg/kg) plus abciximab perfusion (10 µg/min) for 12 h. A 35% reduction was obtained in the primary endpoint (combined death, non-fatal AMI, need for percutaneous or surgical revascularization or failure of the procedure) in the patients treated with bolus plus abciximab perfusion versus placebo (8.3% vs 12.8%; P=.008), mainly due to better non-fatal AMI rates (5.2% vs 8.6%; P=.03) and especially regarding the need for revascularization (0.8% vs 4.5%; P <.001).25 No benefit was observed with abciximab bolus only without perfusion. It should be pointed out, however, that hemorrhages were twice as frequent (14% vs 7%) in the abciximab group versus placebo, which has been attributed to the high-dose heparin regimen unadjusted for weight used in the study.
The EPILOG study evaluated the results of 3 treatment options for low-risk patients undergoing angioplasty: ASA plus standard-dose heparin adjusted for weight (100 U/kg to obtain an activated coagulation time [ACT]>300 s) plus placebo; ASA plus the same dose of heparin plus abciximab; and ASA plus low-dose heparin adjusted for weight (70 U/kg and ACT>200 s) plus abciximab. The incidence of major clinical events at 30 days (death, AMI, or emergency revascularization) was smaller in the groups that received abciximab: 11.7% for the placebo group, 5.4% for the abciximab plus standard-dose heparin group (P<.001), and 5.2% for the abciximab plus low-dose heparin group (P<.001).26 The incidence of serious hemorrhages was similar in the 3 treatment arms.
The EPISTENT study was the first to evaluate the benefit of abciximab in patients undergoing emergency or elective revascularization with stenting. Some 2399 patients with coronary heart disease were randomized to stenting plus placebo, angioplasty plus abciximab, or stenting plus abciximab, all receiving unfractionated heparin (UH) at 100 U/kg. The most favorable outcomes were found in the group of patients assigned to stenting plus abciximab (5.3% major clinical events at 30 days vs 6.9% in the angioplasty plus abciximab group [P=.007] and 10.8% in the stenting plus placebo group [P<.001]). The long-term evolution of these patients continued to show favorable results with abciximab, both at 6 months (lower revascularization rate, with maximum benefit in the diabetic patients) and at 1-year follow-up (reduced mortality rates in the stenting plus placebo group, 1% vs 2.4%; P=.03).29 There were no differences in major hemorrhage rates between the 3 groups.29
The benefit of treatment with abciximab at 6 months in the patients with non-ST segment elevation ACS has been evaluated in the EPIC,30 EPILOG,31 and EPISTENT32 long-term follow-up studies, which demonstrated, as in ST-segment elevated AMI, a reduction in the need for revascularization. However, the role of abciximab in the prevention of coronary restenosis is controversial, as is the case with the other GP IIb/IIIa inhibitors. It is believed that these outcomes are the result of the reduction in acute ischemic events.33,34
Eptifibatide
Eptifibatide (Integrilin®) is a designed cyclic heptapeptide similar to barbourin (poison from the snake Sisfrurus milarus barbouri) which is highly specific and selective for GP IIb/IIIa receptors. Unlike abciximab, it does not have any affinity for other integrin receptors. Its main advantage compared to the former is its more prolonged action and low cost.
The IMPACT-II study was the first large study on eptifibatide in the PCI context, but did not demonstrate benefits regarding the primary endpoint (death, AMI, or need for revascularization at 30 days) versus the placebo group.35 This has been attributed to the low dose at which the drug was used. In fact, later studies with higher doses have demonstrated their efficacy in low-risk patients undergoing cardiac catheterization. The PURSUIT study included almost 11 000 patients with unstable angina, who were randomized to placebo, low-dose eptifibatide (180μg/kg bolus followed by an infusion at 1.3 μg/kg/min), or high-dose eptifibatide (180 μg/kg bolus followed by infusion at 2 μg/kg/min). The group that received high doses of the drug presented lower death or heart attack rates at 30 days than the placebo group (14.2% vs 15.7%; P=.042),36 a difference that was already present from 96 h post-catheterization onwards. Major hemorrhage rates (defined by the TIMI scale) were more frequent in the eptifibatide group (10.6% vs 9.1%; P=.02). The most striking results with eptifibatide, however, were obtained with yet higher doses (180 μg/kg bolus followed by infusion at 2 μg/kg/min plus a new 180 μg/kg bolus 10 min after the first one). Using this line of treatment (together with low-dose heparin: 60 U/kg, ASA and clopidogrel), the ESPRIT study,37 with little more than 2000 patients who were candidates for elective catheterization, demonstrated a 37% reduction in major clinical events at 48 h (6.6% vs 10.5% in the placebo group; P=.0015). This benefit was maintained at 30 days and even at 1-year follow-up, as a recent analysis demonstrated (8% vs 12.4% in the placebo group for combined infarction or death, and 17.5% vs 22.1% for combined death, reinfarction, or need for revascularization at 12 months).38 The prevalence of major hemorrhages, although infrequent, was clearer in the eptifibatide group than in the placebo group (1.3% vs 0.4%; P=.02). On the basis of these results, the treatment regimen used in the ESPRIT study is the one accepted today for eptifibatide.
Tirofiban
Tirofiban (Agrastat®) is a non-peptide GP IIb/IIIa inhibitor with dose-dependent action and high specificity. Like eptifibatide, it has a longer half-life and costs three times less than abciximab.
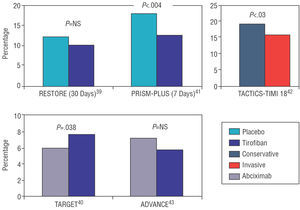
The initial studies with tirofiban in PCI did not yield such positive results as with other GP IIb/IIIa inhibitors. Figure 3 presents the results of the main studies on tirofiban. The RESTORE study was the first to evaluate the efficacy of tirofiban. Some 2139 patients were randomized to treatment with ASA plus heparin plus tirofiban (10 μg/kg bolus followed by infusion at 0.15 μg/kg/min) or treatment with ASA plus heparin plus placebo. Although a significant benefit was observed with tirofiban at 48 h following catheterization and at 7 days, the primary endpoint of the study (combined death, AMI, revascularization surgery or need for new angioplasty at 30 days) did not demonstrate significant differences between the 2 treatment arms, although a favorable trend was observed in the tirofiban group (10.3% vs 12.2%; P =.16).39 Neither were there significant differences in hemorrhage rates between groups. On the other hand, a comparative study of the same doses of tirofiban versus abciximab showed the superiority of abciximab regarding the major clinical events rate at 30 days (6% vs 7.6% for combined death, infarction, or need for emergency revascularization; P =.038), although the lowest incidence of hemorrhages was in the tirofiban group.40 This advantage was consistent regardless of age, sex, the presence of diabetes, or treatment with clopidogrel. Major hemorrhages were similar in the 2 groups, although the tirofiban group presented the lowest incidence of minor hemorrhages.40
Figure 3. Results of the primary endpoints with tirofiban.
These results have not been confirmed by other studies. The PRISM-BONUS study demonstrated in patients with intermediate- and high-risk ACS a reduction in combined death, AMI or refractory ischemia at 7 days in the ASA plus heparin plus tirofiban group versus the ASA plus heparin plus placebo (12.9% vs 17.9%; P=.004)41; and this benefit was maintained at 48 h, 30 days, and 6 months. In addition, in the subgroup of patients undergoing angioplasty, the use of tirofiban combined with ASA and heparin improved the angiographic results, reducing the quantity of intracoronary thrombus by 23% more than in the placebo group. This explains the results in this subgroup of patients, where treatment with tirofiban reduced risk of death, AMI, or refractory angina by 32%, and risk of death or AMI by 43%. On the other hand, the TACTICS-TIMI study18 established the superiority of an early invasive strategy versus conservative treatment in the patients with non-ST segment elevation ACS treated with tirofiban. Incidence of death, AMI, or rehospitalization for refractory angina at 6 months was 15.9% in the tirofiban plus invasive treatment group versus 19.4% in the tirofiban plus conservative treatment group (P=.025).42 There was maximum benefit in the high-risk patients as defined by the TIMI Risk Score scale (TIMI 5-7).
It is believed that the discouraging results of the RESTORE and TARGET studies could be due to the tirofiban dose used which is considered to be insufficient.43 In fact, a new study used higher doses of the drug demonstrating an increase in its efficacy without a greater incidence of hemorrhages. In this study, tirofiban was administered as a 25 μg/kg bolus followed by infusion at 0.15 μg/kg/min for 18 h, and was compared to standard-dose abciximab. The incidence of major ischemic complications was 5.8% in the tirofiban group versus 7.1% in the abciximab group (P=.65), without differences in the hemorrhage rate between the 2 groups.44
ANTITHROMBOTIC DRUGS (II): ANTICOAGULANT DRUGS
Unfractionated Heparin
Unfractionated heparin (UH) is made up of a mixture of glycosaminoglycan polymers with a high variable molecular weight (5-30 kD). Its greatest anticoagulant activity derives from a single pentasaccharide sequence with great affinity for antithrombin III (AT III). The bonding of pentasaccharide to AT III causes a structural change in the latter that strengthens its inhibiting activity on coagulation factors IIa (thrombin), IXa, Xa, and XIa (Figure 1). Of these, thrombin is the factor most sensitive to the effect of heparin, but it requires a minimum-length saccharide chain (18 saccharides) so that this can be combined at the same time with AT III and thrombin and achieve its anticoagulant effect at this level. The great variability in the length of the saccharide chains that form heparin, together with its different ways of bonding to plasma proteins, explains the relative unpredictability of its anticoagulant effect.
Despite these drawbacks, heparin continues to be the standard anticoagulant treatment during PCI, since its price is less than other anticoagulants and, more importantly, its effect can be easily reversed and monitored. The use of heparin during coronary catheterization began in a relatively empirical manner in an attempt at preventing acute cardiac and vascular ischemic complications (formation of thrombi at vascular access points or in the guides and catheters).45 Initially, fixed doses of 10 000 U in bolus with a new later bolus were used in all the patients, depending on the duration of the procedure, which reduced acute occlusion of the vessel and the incidence of early ischemic complications. Subsequently, given the frequency of hemorrhagic complications, administration regimens were initiated adjusted to weight with intraprocedural monitoring via ACT monitoring, taking as reference values those used for coronary revascularization surgery (ACT 300-400 s). Several studies initially demonstrated a reduction in periprocedural ischemic complications with higher ACT values, but this benefit was offset by a higher rate of hemorrhages.
Currently, ACT continues to be the standard monitoring system during PCI, given that the levels of anticoagulation needed exceed the reference range of the activated partial thromboplastin time. There is some controversy regarding the optimum values. Whereas some small studies recommend low-dose heparin (5000 U bolus) for stable patients, obtaining an incidence of acute ischemic complications similar to that of patients treated with high-dose heparin,46,47 a recent metaanalysis reported maximum benefit with high ACT values (350-375 s), as long as there is no concomitant treatment with GP IIb/IIIa inhibitors.48 In general, given the currently available data, in the absence of treatment with GP IIb/IIIa inhibitors, an initial bolus of 70-100 U/kg is recommended with later supplements if necessary (2000-5000 U) to obtain a 300-350 s ACT (for the Hemochrom system) or 250-300 s ACT (for the Hemotech system).22 When treatment is associated with GP IIb/IIIa inhibitors, the heparin dose should be lower: 50-70 U/kg bolus and target ACT>200 s.
The systematic use of heparin after catheterization is not indicated, since no benefits have been demonstrated regarding the incidence of acute ischemic complications or in the restenosis rate. On the other hand, it has been associated with a greater rate of hemorrages.49
Low-Molecular-Weight Heparins
Low-molecular-weight heparins (LMWH) derive from the chemical or enzymatic depolymerization of UH. The majority of the LMWH chains do not have the AT III pentasaccharide binding unit, nor do they contain the minimum 18 saccharides which bind to thrombin that UH has, in such a way that the action of LMWH is basically anti-Xa in contrast to UH which has anti-IIa and anti-Xa 1:1 activity (Figure 1). Low-molecular-weight heparins have some advantages compared to UH, basically due to their powerful anticoagulant effect, a predictable response which makes monitoring unnecessary, the low incidence of thrombocytopenia, and the absence of a rebound hypercoagulation effect. To evaluate the degree of anticoagulation obtained with LMWH, anti-Xa activity values can be measured in blood. A recent study suggests that anti-Xa activity below 0.6 U/mL is associated with a greater incidence of ischemic complications during PCI,50 such that this is considered the minimum degree of anticoagulation required in PCI, although measuring this is complex and is not normally done.
Most studies on LMWH in PCI focus on enoxaparin, although there are also data on other compounds. The REDUCE study was the first to evaluate the effectiveness of LMWH during angioplasty without stenting; 625 patients were randomized to reviparin (7000 U intravenous bolus followed by infusion of 10 500 U over 24 h, and subsequently 3500 U/12 h subcutaneously for 28 days) or UH (10 000 U intravenous bolus followed by infusion of 24 000 U over 24 h). A 52% reduction in the incidence of ischemic events in the first 24 h was obtained with LMWH (P=.027). However, there were no differences between the two groups regarding prognosis at 30 weeks.51 Furthermore, hemorrhagic complications were similar in both groups.
Enoxaparin is the LMWH that has most clearly demonstrated its efficacy in the PCI context, when administered intravenously or subcutaneously. In the NICE-1 study, 828 patients received 1 mg/kg of enoxaparin intravenously prior to the procedure, obtaining an incidence of ischemic events at 30 days of 7.7%,52 which was a similar or even more favorable result than the one obtained in a previous study of comparable patients treated with UH (EPISTENT study,29 placebo group plus stenting). Lower doses (0.5 mg/kg intravenously) have been tried in patients undergoing elective catheterization with good results, although in small samples of patients.53
Similarly, enoxaparin has also demonstrated its efficacy in combination with GP IIb/IIIa inhibitors, mainly administered at a dose of 0.75 mg/kg intravenously. The combination of enoxaparin (0.75 mg/kg) and standard-dose abciximab demonstrated its safety and efficacy in the NICE-4 study.52 This treatment regime yielded an ischemic complication rate similar to that obtained in other studies with abciximab plus UH27,29 with a 0.2% incidence of hemorrhages. Enoxaparin combined with eptifibatide has been compared with UH in a study with 261 patients undergoing urgent or elective catheterization (the CRUISE study).54 There were no significant differences in clinical events between eptifibatide plus enoxaparin group and the eptifibatide plus UH group (8.7% vs 7.6%; P=NS); the hemorrhagic complication rate was also similar in both groups.54
The administration of LMWH subcutaneously in patients undergoing cardiac catheterization has also been proven effective and safe according to the data extracted from certain small studies. In a retrospective analysis of the patients included in the ESSENCE study in France, Collet et al55 obtained a low rate of major cardiac complications (3%) and hemorrhages (0.8%) at 30 days for the patients with unstable angina treated with subcutaneous enoxaparin. Similarly, the NICE-3 study yielded an ischemic complication rate of 7.4% at 30 days for a population of patients with ACS.56 In any case, subcutaneous enoxaparin treatment in patients referred for cardiac catheterization is considered effective whenever it is administered at most 8 h prior to the procedure, since it is in this period that anti-Xa activity values are kept higher than 0.6 U/mL (LMWH effective dose limit, as mentioned previously). When catheterization is done after 8 h, it is accepted that an additional 0.3 mg/kg bolus of enoxaparin should be administered intravenously at the time of carrying out the procedure.5
There are few data on dalteparin in PCI. A small study obtained an acceptable ischemic event rate at 1 month with an intravenous dose of 60 U/kg combined with abciximab.50 When administered subcutaneously, a dose of 120 U/kg should be supplemented with an intravenous 40 U/kg bolus when catheterization is done after 8 h.50
Vitamin K Antagonists
Vitamin K antagonists (better known as oral anticoagulants [OAC]) act by inhibiting the vitamin K activity necessary for synthesis of coagulation factors II, VII, IX, and X in the liver (Figure 1).
Treatment with vitamin K antagonists (warfarin and acenocoumarol are the most used) has been tried in PCI with two main aims: on the one hand, to reduce acute ischemic complications due to early occlusion of the vessel and, on the other, to diminish the incidence of restenosis. Regarding the former, combined ACO/ASA treatment has not demonstrated its superiority versus ASA only and, in any case, has yielded much poorer results than ASA plus thienopyridine.7 For the prevention of postimplantation coronary stent restenosis, treatment with ACO has failed to demonstrate any benefit and, in addition, has been related to an increase in hemorrhagic complications as can be concluded from the 5 main studies that have researched this aspect.5
Thus, treatment with ACO is not currently recommended and its use is even advised against during PCI unless there is another indication.5
Direct Thrombin Inhibitors: Hirudin and Derivatives
Hirudin is a 65 amino acid protein isolated from the salivary glands of the leech (Hirudo medicinalis) which directly and irreversibly combines with thrombin, deactivating its platelet aggregation activity, the coagulation cascade (factors V and VIII), and fibrinogen (Figure 1). Identification of the hirudin structure has made it possible to obtain recombinant forms (bivalirudin) and synthetic agents (argotroban) that contain the N-terminal sequence which neutralizes thrombin.
Hirudin and its derivatives offer certain advantages compared to classic anticoagulants. Unlike heparin, the hirulogs act not only on free thrombin, but also on thrombin that is found within the thrombus, which potentiates its anticoagulant activity. Unlike the vitamin K antagonist anticoagulants, it is fast-acting and has a short half-life (25 min). All the compounds have a predictable dose-response curve and do not produce antibody-mediated thrombocytopenia.
These characteristics have given rise to much hope. In the PCI context, three compounds have been mainly tried: hirudin, bivalirudin (the most studied), and argatroban.
Hirudin was evaluated versus UH in a study with more than 1000 patients with unstable angina undergoing cardiac catheterization. The patients were randomized to three treatment regimens: unfractionated heparin in bolus (10 000 U) plus infusion for 24 h; hirudin in bolus (40 mg) followed by infusion for 24 h; and hirudin in bolus (40 mg) followed by infusion for 24 h plus 40 mg/12 h subcutaneously for 3 days. Hirudin treatment reduced the postprocedural ischemic complications rate by 39% (11% vs 7.9% vs 5.6%, respectively; P=.02), but the primary endpoint of the study, incidence of restenosis at 7 months, was similar in the three groups (67.3%, 63.5%, and 68%, respectively).57
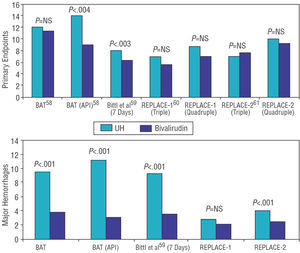
On the other hand, bivalirudin versus UH has been tested in various studies, both in isolation and combined with GP IIb/IIIa inhibitors. However, it must be emphasized that all these studies used very high doses of UH, which considerably increased the incidence of hemorrhages and could have biased the results (Figure 4). The BAT study randomized more than 4000 patients with unstable angina to UH (175 mg/kg bolus followed by perfusion at 15 U/kg/h for 18-24 h) or to bivalirudin (1 mg/kg bolus followed by perfusion at 2.5 mg/kg/h for 4 h and, subsequently, 0.2 mg/kg/h for the following 14-20 h). No differences were found in the bivalirudin group regarding the primary endpoint (combined death, AMI, acute occlusion of the vessel or clinical deterioration of cardiac origin during admission), but there was a lower incidence of hemorrhagic complications (3.8% vs 9.8% in the UH group; P<.00158). However, in the postinfarction angina subgroup, bivalirudin treatment reduced the ischemic complications rate (9.1% vs 14.2%; P=.04) and hemorrhages (3% vs 11.1%; P<.001),58 a benefit that was not maintained at 6 months. In a later intention-to-treat analysis, a 22% reduction was found in combined death, AMI or need for revascularization at 7 days with bivalirudin treatment; in this case the benefit was maintained at 90 and 180 days.59
Figure 4. Main studies comparing bivalirudin versus unfractionated heparin in the context of percutaneous coronary intervention. The results of the primary endpoints and hemorrhagic complications are shown. UH indicates unfractionated heparin.
In combination with GP IIb/IIIa inhibitors, bivalirudin has generally proved to be as good as UH plus GP IIb/IIIa inhibitors, although it has a lower hemorrhage rate (some of which, as mentioned, are related to the high doses of heparin used). The REPLACE-160 and REPLACE-261 studies have researched this aspect in more depth. Patients undergoing elective or emergency catheterization were openly randomized to heparin in bolus (60-70 U/kg in the REPLACE-1 study and 65 U/kg in the REPLACE-2 study) versus bivalirudin in bolus plus perfusion (0.75 mg/kg followed by 1.75 mg/kg/h). The use of GP IIb/IIIa inhibitors in the REPLACE-1 study was at the discretion of the researcher (72% of the patients, which was similar in both groups), whereas in the REPLACE-2 study this was constant in the heparin group and only provisional in the bivalirudin group (7.2% of the patients). None of them demonstrated significant differences between the 2 groups regarding principal endpoints (triple combination of death, AMI or need for revascularization at 30 days and quadruple combination of the former plus major hemorrhage). The incidence of major hemorrhages measured in isolation was, however, lower in the groups that received bivalirudin.
The doses accepted for bivalirudin are those used in the REPLACE-1 and REPLACE-2 studies (0.75 mg in bolus followed by infusion at 1.75 mg/kg/h). In general, treatment with bivalirudin is accepted as anticoagulant therapy during cardiac catheterization, preferably without GP IIb/IIIa inhibitors,5 although its use is not very well established in daily clinical practice. Similarly, bivalirudin can be a good alternative to UH in patients with a high risk of hemorrhage treated with GP IIb/IIIa inhibitors.
Argatroban has not been rigorously evaluated in PCI. It has been successfully used in patients with heparin-induced thrombocytopenia, which means that this can be a valid indication for this drug.62
Recently, a new direct thrombin-inhibiting compound, ximelagatran, has become available which is administered orally, unlike the previous ones. It is rapidly metabolized into melagatran, its active form, independently of cytochrome P450, such that it has few interactions with other drugs. Ximelagatran has been tested in the treatment of deep vein thrombosis and pulmonary thromboembolism. In the context of ischemic heart disease, and combined with ASA, it has demonstrated reductions in the incidence of major clinical events at 6 months in the patients who presented AMI with or without ST segment elevation but who did not undergo cardiac catheterization (12.7% vs 16.3% in the ASA-only treatment group; P=.03), without increasing hemorrhagic complications.63 However, there still are no data regarding the role of ximelagatran in PCI.
Pentasaccharides
Fondaparinux is a recently introduced pentasaccharide that irreversibly inhibits activated factor X with greater specificity than UH or LMWH (Figure 1). It does not interact with platelet factor 4, and thus does not induce thrombocytopenia. It does not need to be monitored. As it has a half-life of 15 h it can only be administered on a daily basis. Factor VIIa reverses its effect.
Fondaparinux has demonstrated its efficacy in the prevention and treatment of thrombosis after orthopedic surgery or in the treatment of pulmonary thromboembolism. In ST-segment elevation AMI, fondaparinux treatment has proved to be as efficacious as UH, and there was even a trend toward less reocclusion of the infarct-related vessel.64 In non-ST-segment elevation acute myocardial infarction, fondaparinux has demonstrated efficacy equal to enoxaparin in the prevention of major cardiac events (death, infarction need for revascularization) at 9 days.65 At a dose of 2.5 mg/day, it has obtained even better results than enoxaparin (27% vs 35.7%; P <.05) with the same endpoint.65 However, in these studies the patients were not undergoing cardiac catheterization; thus, at present, we have few data concerning the usefulness of fondaparinux in PCI.
DRUGS AIMED AT REDUCING THE INCIDENCE OF RESTENOSIS
Oral Sirolimus
Even though the introduction of standard stents made it possible to reduce the incidence of coronary restenosis, this continues to be a serious problem in PCI. The recent appearance of drug-eluting stents (mainly sirolimus and paclitaxel) has made it possible to obtain very favorable results in this context (up to 70%-80% reduction in coronary restenosis at 6 months).20,21
Sirolimus is a macrocyclic lactone with powerful immunosuppressive activity used for the prevention of acute rejection in kidney transplants. Its antiproliferative and antimigratory action, basically demonstrated in experimental models,66 has aroused interest in this drug regarding coronary heart disease in relation to both ischemic heart disease and in cardiac allograft vasculopathy. The results described to date regarding local drug delivery with sirolimus-eluting stents have been very positive in the reduction of coronary restenosis (5.9% vs 42.3% in binary restenosis at 8 months in the standard stent group).20 Based on these figures, several studies have recently appeared in which the effect of oral sirolimus has been tested, with the hypothesis that systemic treatment could offer a benefit similar to that described for local sirolimus. To date, the results obtained are limited and also contradictory. Brara et al67 described the first results in 22 patients with a high risk of coronary restenosis (history of previous restenosis or failed brachytherapy), compared to those administered with 6 mg oral sirolimus in bolus immediately after percutaneous revascularization, followed by 2 mg/day for a month. An 86.7% restenosis rate was obtained, with a revascularization rate of 59.1% in the culprit vessel at 6 months. In addition, there was a high incidence of adverse effects due to treatment, which led to stopping it in 50% of the patients, mainly due to severe leukopenia and hypertriglyceridemia.67 In the same line, another study of 15 patients with a single, low-risk lesion (3.4 mm mean vessel diameter) obtained a 6-month restenosis rate of 40% after 1 month of treatment with oral sirolimus.68 These results have not been confirmed by other studies. The OSIRIS study randomized 300 patients to one of three treatment arms: placebo, treatment with standard-dose oral sirolimus (8 mg bolus followed by 2 mg/day for 7 days), or treatment with high-dose sirolimus (24 mg bolus followed by 2 mg/day for 7 days). Treatment with sirolimus was related to an improvement in the incidence of binary restenosis (>50%) at 6 months (22.1% in the high-dose sirolimus group vs 38.6% in the standard-dose sirolimus group, and 42.2% in the placebo group; P=.005), with a non-significant trend toward reduced need for revascularization.69 There were fewer side effects with a 7-day treatment regimen (2.6% of the patients).69
In addition, there is no consensus regarding whether the effect of sirolimus is dose-dependent. In contrast to the stated results of the OSIRIS study, where the greatest benefit was obtained in the high-dose sirolimus group, the ORBIT study describes similar binary restenosis rates in the patients treated with 2 mg/day oral sirolimus for 1 month versus the patients treated with dose of 5 mg/day (7.2% and 6.9%, respectively; P=NS, which are lower values than the ones described for restenosis in the control groups without sirolimus).70 On the other hand, a pilot study with 34 patients indicated that better results were associated with sirolimus blood concentrations higher than 8 ng/mL.71
It can be deduced from all the foregoing that oral sirolimus treatment has not demonstrated a clear benefit to date and, obviously, more studies are needed to establish its role in the prevention of coronary restenosis.
Other Drugs
Other drugs have been tried with the aim to reduce coronary restenosis, generally with little result.
Statins have already been used in the angioplasty era, without demonstrable benefit. On the other hand, a retrospective study of 525 consecutive patients undergoing percutaneous revascularization with stenting demonstrated that the use of statins was related to a lower coronary restenosis rate versus the control group (25.4% vs 38%; P<.005).72 Apparently, the difference in benefit from statins and stenting versus simple angioplasty could be explained by a coronary restenosis process based on more serious intimal hyperplasia due to stenting, for which the antiinflammatory and antithrombotic effect of the statins would be more useful. However, these results with statins have not been confirmed in later studies, at least in ostial lesions,73 and thus the role of the statins for the exclusive prevention of coronary restenosis has not been established.
Finally, some data are available regarding coronary restenosis using ACE inhibitors, but generally with few results of interest. On the other hand, the angiotensin receptor II antagonists seem to have a more important role in the restenosis process, probably due to a stronger effect on reducing circulating cytokines and growth factors, and on reducing neutrophil activation.74 The VAL-PREST study, with 250 patients randomized to treatment with 80 mg/day valsartan or placebo, demonstrated reductions in the intrastent restenosis rate (17% vs 38%; P<.005) and need for revascularization (12.1% vs 28.7%; P<.005) at 6 months in the valsartan group versus the placebo group.75 In any case, this was a small study and there is a need for data on larger populations to definitively establish the benefit of these drugs in the prevention of coronary restenosis.
Section Sponsored by the Dr Esteve Laboratory
Correspondence: Dra. M. Masotti.
Institut Clínic de Malalties Cardiovasculars.
Hospital Clínic. Universidad de Barcelona.
Villarroel, 170. 08036 Barcelona. España.
E-mail: mmasotti@clinic.ub.es