Cases of acute myocarditis have been reported in relation to SARS-CoV-2 infection, and after administration of the first and second dose of the BNT162b2 vaccine1 or a single dose of Ad26.COV2.S.2
We describe the clinical case of a 24-year-old man with Crohn's disease, receiving treatment with adalimumab, which was discontinued on his own account 4 months before hospital admission. He had been vaccinated with the complete regimen (2 doses) of BNT162b2 while off treatment with adalimumab. At the second dose, he noted self-limited chest pain, with no other clinical signs or symptoms.
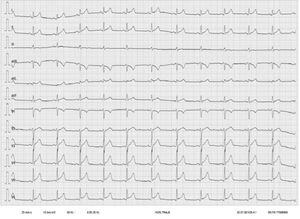
At 24hours after receiving the third dose of BNT162b2, the patient experienced pericardial chest pain and a low-grade fever of 37.6°C.On emergency room admission, the electrocardiogram showed sinus rhythm at 71 bpm and a diffuse, concave ST-segment elevation consistent with acute pericarditis (figure 1). Ultrasensitive troponin I analysis yielded an initial value of 11,183 ng/L (normal, < 34 ng/L), a peak of 17,650 ng/L at 4hours after admission, and a subsequent descending curve that reached 135 ng/L at 5 days. Serological tests for cardiotropic pathogens were negative for IgM, and basic autoimmunity screening was negative.3
Polymerase chain reaction (PCR) testing for SARS-CoV-2 was negative in 2 nasopharyngeal swabs taken 2 days apart. SARS-CoV-2 IgM and IgG serological tests were not available.
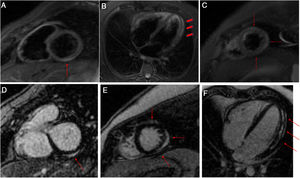
There were no segmental contractility abnormalities or pericardial effusion on transthoracic echocardiography, and the left ventricular (LV) ejection fraction was 56%. The total longitudinal strain showed changes in the basal anterolateral and mid-anterolateral segmental deformation (–14%) in the apical view. The patient remained asymptomatic after starting ibuprofen 600 mg/8h and colchicine 0.5 mg/12 h. Cardiac magnetic resonance imaging showed no segmental contraction abnormalities and preserved LV systolic function (53%). T2-weighted STIR sequences depicted hyperintensities indicative of edema in the basal inferior and lateral segments, midlateral segment, and apical anterior, lateral, and inferior segments. Pericardial thickness was normal and there was no pericardial effusion. Myocardial late gadolinium imaging detected patchy, subepicardial enhancement in the above-mentioned segments (figure 2).
T2-weighted STIR images in short-axis (A and B) and 4-chamber (C) views. Hyperintensities related to edema are seen in the basal inferior and lateral segments, the mid-lateral segment, and the apical anterior, lateral, and inferior segments (arrows and arrowheads). Delayed enhancement images in the short-axis (D and E) and 4-chamber views (F). Patchy late gadolinium enhancement is seen in the subepicardial myocardium (arrows).
Based on these findings, a diagnosis was established of acute myocarditis predominantly affecting the lateral LV wall. The involvement of various myocardial segments corresponding to different coronary territories and the excellent clinical progression with anti-inflammatory treatment in a young patient with low cardiovascular risk led to exclusion of coronary anatomical study and endomyocardial biopsy.
At an outpatient follow-up visit at 34 days, the patient remained asymptomatic under treatment with ibuprofen and colchicine The previous diffuse changes had normalized on electrocardiography, and the total longitudinal strain showed no abnormalities in the total (–19%) or segmental deformation on echocardiography. The patient gave written informed consent for publication of the case.
Patients with inflammatory bowel disease have a higher associated incidence of acute myocarditis than the general population, mainly in the phase of acute disease exacerbation.4
Our patient had no intestinal symptoms at diagnosis of the cardiac condition. The existence of a molecular mimicry mechanism that can lead to immune disorders after vaccination has been hypothesized in genetically predisposed individuals.5 There are no studies describing acute myocarditis in patients with inflammatory bowel disease following any of the SARS-CoV-2 vaccination doses. The present report is the first clinical case.
One case of acute eosinophilic necrotizing myocarditis has been described in a patient receiving adalimumab.6 Our patient showed no evidence of peripheral eosinophilia, and he had discontinued adalimumab treatment.
Although SARS-CoV-2 serologic results were not available, the close temporal relationship with symptom onset after vaccination, the negative SARS-CoV-2 PCR results at 2 different time points, which ruled out infection as the cause of myocarditis, the negative results in the extensive serological and molecular study of cardiotropic pathogens, and the absence of documented autoimmunity, allows us to establish the association between the third dose of the BNT162b2 vaccine and acute myocarditis in our patient.
In summary, we report the first clinical case of acute myocarditis after the third dose of BNT162b2 vaccine, with a benign clinical course and no complications. It is advisable to investigate this diagnosis in patients with compatible symptoms after the third dose of BNT162b2, even if no adverse reactions occurred with previous doses.
FUNDINGThere was no funding for this report.
AUTHORS’ CONTRIBUTIONSX. Fosch, the cardiologist responsible for the patient's care during hospitalization and outpatient follow-up, wrote the article and the revisions. J. Serra collaborated in writing and revising the text, and provided the patient with clinical support during hospitalization. P.L. Torres, a cardiologist, collaborated in the patient's treatment during hospitalization. L. Pedrea and R. González were responsible for accepting, performing, and interpreting the cardiac magnetic resonance images. These authors provided the specific sequences and views referred to in the text and the descriptive legends. F. Mojer, coordinator of the medical area, collaborated in the clinical treatment.
CONFLICTS OF INTERESTAll authors declare no conflicts of interest.