To the Editor,
Cardiac tumors are an unusual cause of acute limb ischemia. Myxomas are the most frequent benign tumors and most are symptomatic. Embolization occurs in 30% to 40% of patients with myxomas, with cerebral arteries being the most common destination.1
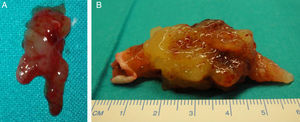
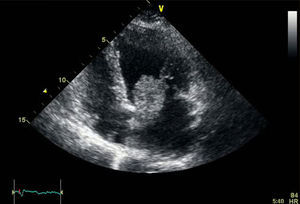
We present the case of a 73-year-old woman with a history of hypertension who came to the emergency department due to pain and sudden onset of coldness in the left limb, which developed over 3h. On examination, she had a brachial pulse in the bicipital groove, without distal pulse or deficits in sensitivity and motility. Cardiac auscultation was normal and the electrocardiogram showed no abnormalities in rhythm, just an incomplete blockage of the right branch. With the diagnosis of acute limb ischemia, the patient was urgently operated on. A thrombectomy of the brachial, radial, and ulnar artery was performed, obtaining abundant material that was gelatinous and friable to the touch, very different from a typical thrombus (Figure 1A). After surgery, the patient recovered distal pulses. Suspecting a cardiac tumor, an urgent transthoracic echocardiogram was ordered, which showed a mobile mass with a wide attachment base, anchored to the interatrial septum. The tumor was approximately 36mm × 25mm, which prolapsed into the left ventricle, producing probable traumatic severe mitral regurgitation associated with the traction of the anterior mitral leaflet (Figure 2). The size and function of the left ventricle were normal. These findings were confirmed by a transesophageal echocardiogram. The study was completed with a cardiac catheterization that resulted in a 70% to 80% stenosis in the middle part of the anterior descending artery. After anesthesia assessment, the patient was taken to the cardiac surgery department for intervention. We proceeded with bi-atrial resection of the tumor with coronary bypass of the left mammary artery to the anterior descending artery, and mitral valve repair using annuloplasty with Edwards ring. The resected tumor was 55mm × 40mm (Figure 1B), and the interatrial septum defect was repaired with a bovine pericardium patch. The diagnosis of myxoma was confirmed after pathological study of the tumor. After 1 year of follow-up, the patient was asymptomatic and had no relapses.
Figure 1. A, embolus extracted from the brachial bifurcation of the arm. B, image of the resected tumor.
Figure 2. Image of the transthoracic echocardiogram that shows a tumor attached to the septum prolapsing into the ventricle.
Myxomas originate in the mesenchymal cells in the endocardium of the septum. They have an incidence rate of 0.5% per million inhabitants/year. They are more frequent in women by a 3:1 margin. Most are located in the left atrium (87%) in the septum near the fossa ovalis. Other less frequent locations include the mitral valve, the right atrium, and the ventricles.2
There are familial forms, called the Carney complex, that are inherited in an autosomal dominant manner, characterized by mesenchymal tumours, cutaneous lentiginosis, hyperfunctional endocrine disorders, and tumors of the peripheral nerves.3 Symptoms depend on location, size, and mobility of the tumor. Symptoms may be due to obstruction of the ventricle, with the onset of dyspnea, secondary to pulmonary edema, syncope, and even sudden death. Systemic embolization occurs in 25% to 40% of cases due to tumor fragmentation, with the brain being the most frequent destination. Limb embolisms, as in our case, in visceral, coronary, and renal arteries are rare. Complete embolization of the tumor has also been reported. Patients may present with symptoms including fever, skin rash, joint pain, and weight loss, as a result of the production of cytokines (interleukin-6) and growth factors by the myxoma.4
Transthoracic echocardiography is the method of choice for the diagnosis of cardiac myxomas. However, transesophageal ultrasound has greater sensitivity (almost 100%) than transthoracic ultrasound (90%).5 The treatment of choice is surgical resection even in asymptomatic patients. It must be performed very soon after diagnosis due to the risk of embolism and sudden death. Surgical mortality varies from 2% to 5%, and the prognosis after resection is excellent. Recurrence of these tumors varies from 0.4% to 5% at 22 years of treatment. Therefore, follow-up with echocardiography is recommended at least every 5 years. Cardiac myxomas have greater recurrence in young males, multifocal cases, and familial forms.6 In these patients, follow-up must therefore be more stringent.
Corresponding author: acuariuss4@hotmail.com