Myotonic dystrophy type I, or Steinert disease, is a multisystemic disease. We present the case of a patient who, after ophthalmologic examination and instillation of atropine eye drops, developed wide QRS complex tachycardia as the first manifestation of Steinert disease.
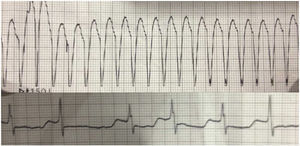
The patient was a 52-year-old woman who had undergone gallbladder removal because of biliary pancreatitis. She had been followed up for 12 years because of cataracts but had no other history of interest or treatment. After topical atropine installation in an ophthalmologic examination, she developed rapid palpitations, poor general status, and chest pain. Sustained monomorphic broad-complex tachycardia (Figure) was observed in the emergency department, together with hypertension, and hemodynamic angina. She was sedated with propofol, and synchronized electrical cardioversion with 150J, proved to be effective, triggering atrial fibrillation and controlled ventricular response, which reverted to sinus rhythm with amiodarone. The maximum troponin level was 15.01 ng/mL (normal values, < 0.056 ng/mL) and creatine kinase was 522 U/L (normal values, 29 – 192 U/L). The patient was stabilized in the hospital intensive care unit and, once in the inpatient medical ward, bilateral palpebral ptosis, muscular weakness, temporal muscle atrophy, thenar and hypothenar eminences, and myotonic phenomenon were observed. The baseline electrocardiogram showed sinus rhythm at 60 bpm, with a long PR interval of 210ms, with a QRS of 100ms shown by left axis deviation, with left anterior hemiblock, and QT corrected to 490ms, probably in the setting of myocardial ischemia due to poor tolerance of the tachycardia and amiodarone use. Once the drug was discontinued and the acute phase had passed, the abnormalities resolved. Echocardiography and magnetic resonance angiography showed no structural heart disease. Computed tomography angiography demonstrated normal coronary arteries on 24-hour Holter monitoring and there were no signs of tachycardia. We requested an electromyogram, which confirmed that the signs of myopathy and myotonic discharges were predominantly of the distal musculature. The patient was referred to the genetics unit, which confirmed the diagnosis of Steinert disease.
Steinert disease is the most common myotonic dystrophy, with a prevalence of 0.5 and 18.1 per 100 000 population.1 Inheritance is autosomal dominant with anticipation. The disease is produced by the abnormally elevated repetition (> 35 repetitions) of the cytosine-thymine-guanine triplet in region 13 of the long arm of chromosome 19 (19q13).2 Patients show variable degrees of muscle weakness in addition to ocular involvement (in the form of cataracts), plus gastrointestinal and endocrine involvement, among others. The most common complications are respiratory, derived from pharyngeal and esophageal weakness and myotonic dystrophy, as well as abnormalities of the respiratory muscles and respiratory centers.
The hearts of patients with Steinert disease show fibrosis, atrophy, and degenerative changes, especially in the conduction system, which are more common in the atrioventricular bundle of His.3 The most frequent cardiac manifestations are arrhythmic disorders. The most common is atrioventricular block, appearing in up to 30% of the patients. Ventricular arrhythmias are rare, occurring in around 5% of the patients, and with ventricular dysfunction (in 1.3%).4 In the case of ventricular arrhythmias, the most common mechanism is bundle branch block, which has been reported in 100% of patients with Steinert disease and associated ventricular tachycardias.5 In bundle branch reentry, the depolarizing wavefront travels through one of the branches of the bundle of His in an antegrade direction and, from the ventricles, travels through the other branch in an retrograde direction to again reach the bifurcation of the bundle of His. The most frequent form of bundle branch reentry is type 1, in which the wavefront travels through the right branch in an antegrade direction and through the left branch in a retrograde direction to reach the His bundle; there is also type II bundle branch reentry in which the circuit is the reverse. To be triggered, the reentry mechanism requires a slow conduction pathway, with a short refractory period, and another rapid conduction pathway, with a longer refractory period. In the case presented here, the presence of the conduction disorder in the left branch and the modification of the refractory periods of the branches induced by the atropine, could have created the conditions necessary to establish a reentry between the 2 branches of the His bundle. However, because of the absence of an electrophysiological study, we cannot be sure that this was the causative mechanism. We have found no reports of cases similar to that reported here, although cases of wide QRS tachycardia have been reported following intravenous atropine administration during stress echocardiography with dobutamine.6 There are, however, previous reports of the potential systemic cardiac effects of eye drops. Consequently, and given the infrequency of wide QRS complex tachycardia in patients with Steinert disease and its temporal relationship with atropine administration, we believe that that the eye drop administration was related to the onset of tachycardia in our patient, who was predisposed to this dysrhythmia by her underlying disease and the conduction disorder in the left branch of the bundle of His.