Amniotic fluid embolism (AFE) is a rare obstetric complication. The clinical presentation varies from moderate organ dysfunction to cardiogenic shock, respiratory failure, disseminated intravascular coagulation, and death in 60% of patients. The treatment for AFE is based on cardiopulmonary support and correction of the coagulopathy.1
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) has been used in patients with AFE who have developed cardiogenic shock and respiratory failure,2 although this treatment is not always sufficient. We present the first published case of a patient with a clinical presentation compatible with AFE who required cardiopulmonary support with VA-ECMO and left ventricular assistance with Impella CP.
A 34-year-old woman, gravida 2 with 1 previous cesarean delivery, was admitted in week 38 of her pregnancy due to premature rupture of membranes. On blood tests, hemoglobin was 12.1g/dL; platelets, 180 000/μL; prothrombin time, 96%; international normalized ratio, 1.02; activated partial thromboplastin time, 24.8seconds; and fibrinogen, 334mg/dL. Four hours after admission, urgent cesarean was performed due to fetal bradycardia. Toward the end of surgery, she had diffuse capillary bleeding and uterine atony with hemodynamic and respiratory compromise, for which she received fluid resuscitation, vasopressors, uterotonic drugs, mechanical ventilation, and transfusion of blood products. The patient then went into pulseless electrical activity, with recovery of circulation after 7 minutes of advanced life support.
She was subsequently moved to the intensive care unit on mechanical ventilation, still hemodynamically unstable, with noradrenaline at 0.8μg/kg/min and blood results compatible with disseminated intravascular coagulation (platelets, 26 000/μL; international normalized ratio, 1.67; activated partial thromboplastin time, 75.2seconds; fibrinogen, 81mg/dL; and D-dimer, 88 332 ng/mL). The coagulopathy was corrected with thromboelastography guidance, the noradrenaline dose was reduced, and arteriography was performed that showed active bleeding from both uterine arteries, which were then embolized. At 12hours postadmission, it was decided to proceed to urgent surgery because the patient had ongoing abdominal hemorrhage with significant hemodynamic and respiratory compromise, going into acute pulmonary edema. Transesophageal echocardiography showed severe biventricular dysfunction with a Simpson's left ventricular ejection fraction of 8%.
It was decided to implant a femoro-femoral VA-ECMO (Cardiohelp Maquet Cardiopulmonary; Hirrlingen, Germany) and intra-aortic balloon counterpulsation. Good gas exchange was maintained with VA-ECMO at 4.0-4.5 L/min, but transesophageal echocardiography showed a severely-dilated akinetic left ventricle, unable to open the aortic valve despite high-dose dobutamine and reduction of VA-ECMO flow. An Impella CP was inserted percutaneously via the left femoral artery, after withdrawal of the intra-aortic balloon counterpulsation, to unload the left ventricle. Surgical hemostasis was required to stop bleeding at the entry point.
The patient returned to the intensive care unit with VA-ECMO at 4.0-4.5 L/min and Impella CP at 2.4-2.4 L/min, which allowed gradual reduction of catecholamines. She became oligo-anuric and renal replacement therapy was started with regional calcium citrate anticoagulation.
On day 2 of admission, low-dose intravenous heparin was started to prevent complications associated with the VA-ECMO and Impella CP but, given the ongoing bleeding and life-threatening coagulopathy, it was decided to take a heparin-free approach.
On day 3, the patient was hemodynamically more stable, and transesophageal echocardiography showed improved ventricular function with aortic valve opening, so the Impella CP was removed (after 48 hours’ use) and VA-ECMO was continued due to significant respiratory failure. The patient had a high transfusion requirement and coagulopathy with active bleeding, so was taken to surgery twice more due to hemoperitoneum (days 3 and 4 of admission). On day 4, bleeding was finally controlled, after a total transfusion of 104 units of packed red cells, 66 units of fresh frozen plasma, 30 pools of platelets, 28 g of fibrinogen, 20 g of calcium chloride, 20μg of desmopressin, and 2 doses of 70 g of immunoglobulins.
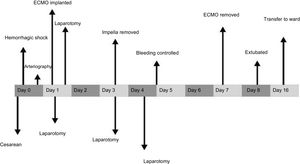
She continued to receive ECMO at 4 L/min for respiratory support, without anticoagulation due to the high bleeding risk, and renal replacement therapy to achieve a negative fluid balance, which led to improved respiratory function and recovery of urine output. The VA-ECMO was removed at day 7 (144hours of use). The patient woke up with no focal neurology; she was extubated with good tolerance at day 8 and was discharged from the intensive care unit at day 16 of admission. A timeline of the patient's clinical course is summarized in Figure 1.
AFE is an obstetric complication that leads to cardiorespiratory failure and coagulopathy. Although VA-ECMO has been used with success in cases of AFE, in our patient this measure was not sufficient to unload the left ventricle, so the Impella CP was added.3 Pappalardo et al.4 recently found that combined therapy with VA-ECMO and Impella can reduce mortality and improve outcomes in patients with cardiogenic shock vs VA-ECMO only. Usually, anticoagulation is imperative for patients with VA-ECMO and Impella CP to avoid circuit thrombosis.3 Given the clotting disorders and active bleeding in this patient, it was decided to take a heparin-free approach4,5 with high-flow VA-ECMO (> 4 L/min) and Impella CP continued at 2.0 to 2.4 L/min to minimize secondary hemolysis, with 20% dextrose being used as the priming solution for the system.
This case report describes for the first time the combined use of VA-ECMO and Impella CP in cardiogenic shock and respiratory failure secondary to AFE in a patient with hemorrhagic shock and associated coagulopathy.