Keywords
INTRODUCTION
Whenever technically possible, valve repair (VR) is the surgical treatment of choice for mitral regurgitation (MR). Among the advantages of this technique over valve replacement is the reduced use of prosthetic material, which lowers the incidence of endocarditis and thromboembolic complications, as well as the need for chronic anticoagulation.1-4 In addition, the subvalvular apparatus is spared, hence postoperative left ventricular function is more highly preserved.4-6 This has resulted in benefits in long-term survival among patients with mitral valve prolapse who undergo VR versus artificial valve placement, without leading to a higher incidence of reoperation.7 Valve repair is also superior to valve replacement in nondegenerative valve disease involving greater surgical risk, such as ischemic MR8,9 and MR secondary to terminal dilated cardiomyopathy.10-12 In 1957, Lillehei et al13 performed the first open mitral annuloplasty under extracorporeal circulation. Since then, a number of surgical techniques to repair the mitral valve have been described and most nonrheumatic cases of MR are now amenable to repair. The purpose of this study was to analyze the mid-term results among patients with chronic MR who have undergone VR at our center over the last 8 years.
PATIENTS AND METHODS
Between January 1997 and July 2004 we performed 74 mitral plasties as elective surgery in patients with chronic MR. Mean patient age was 60±14 years (range, 16-83 years); 59% (n=44) were men. Before surgery, 68% of the patients were New York Heart Association (NYHA) functional class III-IV and 27% were in atrial fibrillation. The mean preoperative left ventricular ejection fraction (LVEF) was 56%, although 16% of the patients (n=12) had severe ventricular dysfunction (LVEF=39%). The etiology of MR was degenerative in 37 (50%) cases, ischemic in 12 (16%), hypertrophic obstructive cardiomyopathy in 11 (15%), dilated cardiomyopathy in 6 (8%), endocarditis sequelae in 4 (5%), and rheumatic in 4 (5%). The pathophysiological mechanism causing MR was most frequently posterior leaflet prolapse (n=29, 39%), followed by central MR due to ring dilation or restrictive motion of the valve leaflets (n=22, 30%), systolic anterior motion (n=11, 15%), mixed prolapse of both leaflets (n=6, 8%), and exclusively anterior leaflet prolapse (n=6, 8%). The type of mitral VR most frequently performed was quadrangular resection of the posterior leaflet in association with ring annuloplasty (n=26, 35%). In 18 (24%) patients, the double-orifice (Alfieri) technique was used; in 10 (56%) of these cases, together with ring annuloplasty. In 18 (24%) patients, anterior leaflet plasty was performed: longitudinal folding plasty (in 11 patients with refractory hypertrophic obstructive cardiomyopathy, MR and systolic anterior motion), implantation of artificial expanded polytetrafluoroethylene chordae (n=3), triangular resection (n=2), excess tissue resection and repair with pericardium patch (n=1), and chordal shortening (n=1). In 50 (68%) patients, a partially flexible ring was implanted (Carpentier-Edwards Physio®, Edwards Lifesciences LLC, Irvine, CA, United States), as a single technique in 15 (30%) of them and additional to other techniques in the remaining 35 (70%). Four (5%) patients underwent Wooler annuloplasty as an alternative to ring annuloplasty. In 37 (50%) patients, the VR was associated with another surgical procedure: coronary revascularization (n=11), extended septal myectomy (n=11), aortic valve replacement (n=7), tricuspid plasty (n=4), aortic valve plasty (n=1), closure of congenital septal defects (n=2), and aortic valve replacement plus myocardial revascularization (n=1).
All patients underwent intraoperative transesophageal echocardiography (pre-VR and post-VR) and a control transthoracic echocardiography at the time of the final clinical follow-up visit. The pre-VR and post-VR quantitative variables were compared by Student's t test for paired data and the survival analysis was done by the Kaplan-Meier method.
RESULTS
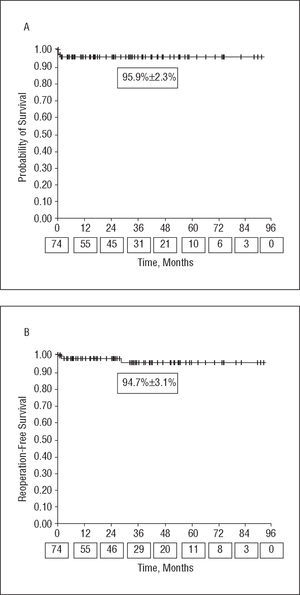
Mean patient follow-up was 38±22 months. A comparison of the preoperative and postoperative clinical and echocardiographic data is shown in Table 1. Both the MR grade and degree of dyspnea decreased significantly after VR, with LVEF with postoperative preservation of LVEF and a decrease in the left ventricular end-diastolic diameter. One (1.3%) patient who had undergone quadrangular resection of the posterior leaflet associated with ring annuloplasty required reentry into extracorporeal circulation due to residual Grade 3 MR in the intraoperative echocardiogram, which was resolved with the double-orifice technique. There was no operative mortality. One patient (1.3%) was reoperated for hemorrhaging and one required intraaortic balloon counterpulsation during the postoperative period. Two patients (2.7%) died while hospitalized (Table 2). Three patients (4%) required reoperation for recurrence of significant MR (Table 3); one of these patients died after valve replacement, the only case of mortality in our series during follow-up (1.3%). Overall survival and reoperation-free survival were 95.9±2.3% and 94.7±3.1% at 3 years, respectively (Figure). At the time of the final clinical follow-up visit, 75% of the patients were not receiving anticoagulants. No hemorrhaging, thromboembolic, or infectious complications were reported during follow-up, although there were 2 cases of ventricular tachycardia that required defibrillator implantation: 1 patient with MR secondary to dilated cardiomyopathy and 40% LVEF and another with posterior leaflet prolapse and a history of syncope.
Figure. Kaplan-Meier survival curves. The values on the x-axis below the time points indicate the number of patients at risk for each follow-up interval. A: overall survival. B: reoperation-free survival.
DISCUSSION
The series we present is not homogeneous and is small in terms of number of patients and follow-up, but it reflects the actual situation of MR and VR in our setting. In Spain, rheumatic disease continues to be the most frequent cause of MR, and therefore the percentage of mitral valves repaired is low as compared to Anglo-Saxon countries, where degenerative valve disease predominates. Nevertheless, MR is a tremendously heterogeneous disease from the etiological, anato-mic, and functional standpoint, making it necessary for the surgeon to be familiar with a wide range of techniques so that each patient can be treated individually, while also making it difficult to enroll patients with similar characteristics.
In our experience, VR has been shown to be an effective surgical option for correcting MR and allowing significant functional recovery for patients. The in-hospital mortality of the series was low (2.7%), particularly if we consider that VR was associated with another type of surgery in almost 50% of the cases and that 16% of the patients had severely depressed LVEF, which has been related to increased operative risk.12,14 The fact that there were no thromboembolic, hemorrhagic, or infectious complications can be attributed to a reduced use of prosthetic material and low percentage of anticoagulated patients after 3 months post-VR (25%). These results are consistent with large VR series, which show a lower incidence of complications and reduced in-hospital mortality when compared to valve replacement.1-4
The mitral subvalvular apparatus contributes to optimizing the inotropic state of the left ventricle, decreasing wall stress and ensuring adequate ventricular geometry. Its preservation is essential to maintain postoperative LVEF and the key to obtaining better results with VR, replacement, and chordal preservation than with conventional replacement.4-6 In our series, LVEF was preserved after surgery and, the significant reduction in left ventricular end-diastolic diameter during follow-up indicates a trend toward regression of ventricular remodeling.
Although this is a MR series of multiple etiology, the mid-term survival of our series (95.9% at 3 years) was good, with the results consistent with those published for degenerative MR by the Mayo Clinic (86% at 5 years).7
The probability of reoperation for MR after VR is high after surgery and later decreases, but there is a slow, gradual rising again over the years.3,15 With the current techniques, the durability of VR is equal to or greater than that of prostheses.4,7 We also reoperated more patients in the first 2 months post-VR than later. The early reoperations were generally required for technical problems (ring dehiscence), whereas late recurrence was due to progression of the degenerative disease. In 2 of the 3 cases reoperated, the prolapse affected the anterior leaflet, which is common in large series, with anterior prolapse an independent predictive risk factor for reoperation.7,15 Although the follow-up lasted to mid-term, durability of the reoperation was good, with 94.7% of the patients reoperation-free 3 years after surgery. Valve repair lasts longest in cases of degenerative MR, and experienced centers report rates of 7% to 11% of valve replacements in the first 10 years after VR due to recurrent MR.3,7,15 In a series with several etiologies similar to ours, Thourani et al4 found a reoperation-free survival of 78% at 10 years, even higher than with valve replacement.
CONCLUSIONS
Valve repair adequately corrects valvular maladaptation and facilitates functional recovery of patients with chronic MR. Since the subvalvular apparatus is preserved, postoperative systolic dysfunction is prevented and ventricular remodeling tends to revert. Hospital morbidity and mortality is low, complications inherent to the prostheses are avoided, and mid-term overall and reoperation-free survival is higher than 90%.
Correspondence: Dr. E. Castedo.
Departamento de Cirugía Cardiovascular. Clínica Puerta de Hierro.
San Martín de Porres, 4. 28035 Madrid. España.
E-mail: evaristocm@terra.es