Keywords
INTRODUCTION
Postoperative right ventricular systolic dysfunction is associated with multiple factors, such as perioperative myocardial ischemia, hypothermic cardiac arrest, pericardial injury and adhesions.1-10 Right ventricular dysfunction after coronary artery bypass graft surgery (CABG) can persist for up to one year.11,12 Right ventricular function following isolated valve surgery has not been extensively studied to date. Pulsed-wave Doppler tissue imaging assessment of tricuspid annular motion is an appropriate and relatively reliable technique for determining right ventricular systolic function.13 Myocardial acceleration during isovolumic contraction (IVA) and isovolumic contraction myocardial velocity (IVV) are less affected by the loading conditions; they therefore predict left and right ventricular functions in a relatively load-independent way.14,15 Myocardial acceleration during isovolumic contraction assessment seems to be a more sensitive technique than tissue Doppler imaging (TDI)-derived peak systolic velocity assessment for predicting contractility.16,17 A recent study has shown the reliability of IVA assessment in the prediction of right ventricular systolic dysfunction in patients with mitral stenosis.18 We investigated the usefulness of pre-operative conventional and tissue Doppler echocardiographic variables in predicting postoperative functional recovery in patients who had undergone isolated valve surgery.
METHODS
Sixty-three consecutive patients who were candidates for isolated valve surgery at Kosuyolu Heart Education and Research Hospital were enrolled in the present study. Patients with a history of significant coronary artery disease (angiographically >50% stenosis, history of acute coronary syndrome or CABG), chronic obstructive or restrictive pulmonary disease, obstructive sleep apnea, congestive heart failure, chronic renal insufficiency (serum creatinine >1.5 mg/dL in men, >1.3 mg/dL in women) or cancer were excluded, as were those for whom the quality of echocardiographic imaging was poor (8 patients). All patients gave informed consent to take part in the study, which was approved by the appropriate institutional review board.
Valve Surgery Procedure
Following median sternotomy, the pericardium was excised and the aorta and superior and inferior vena cava cannulated. Cardiac arrest was achieved with antegrade and retrograde mild hypothermic blood cardioplegia. Mitral valve surgery was performed after left atriotomy and mitral valve exploration. Mechanical mitral valve replacement was performed by the resection of the native valve and replacement with the prosthetic valve using a 2.0 ticron suture. Antegrade blood cardioplegia was used in all mitral valve surgeries. Mitral valve repair was performed in suitable cases. Aortic valve surgery was performed following aortotomy and resection of the native valve. Aortic mechanical valve replacement was performed with continuous retrograde blood cardioplegia following antegrade blood cardioplegia. Combined antegrade and retrograde blood cardioplegia was used in patients who underwent surgery secondary to aortic regurgitation or combined mitral and aortic surgery. All patients were evaluated by echocardiography before and one week after cardiac surgery. Aortic cross-clamp time, operation and pump duration and maximum hypothermia level were obtained from surgery records. All patients were subjected to further echocardiographic studies 1 and 6 months after surgery. Functional recovery was defined as an improvement of at least one NYHA class at sixth months.
Echocardiographic Studies
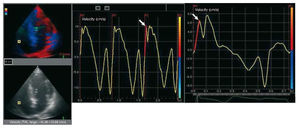
Echocardiographic studies were performed with all patients in the left lateral decubitus position using standard views and employing commercially available equipment (Vivid 5, GE Vingmed, Norway). The left atrial systolic dimension and LV internal dimensions and wall thickness were measured from 2-dimensional guided M-mode echocardiographic tracings obtained at midchordal level in the parasternal long axis view according to the criteria of the American Society of Echocardiography.19 The percentage fractional shortening and ejection fraction were calculated using the Teichholz Formula.20 Color TDI was performed in the apical 4-chamber view employing a 2.5-MHz transducer. Gain settings, filters and pulse repetitive frequency were adjusted to optimize color saturation; a color Doppler frame scanning rate of 100-140 Hz was used. Images were obtained immediately after expiration. Patients were required to hold their breath after expiration for better image quality. All images were digitalized and myocardial regional velocity curves constructed.21 The analysis of tissue Doppler velocity profiles was performed offline using commercially available computer software (Echopac, GE-Vingmed). The myocardial velocity profiles of the basal septal and lateral mitral annulus were obtained by placing a 7´7 mm sample volume at the junction of the mitral annulus with the septum and lateral myocardial wall. Myocardial velocities of the lateral tricuspid annulus were similarly obtained by placing the sample volume at the junction of the tricuspid valve annulus and the RV free wall (RVs). Peak septal and lateral mitral annular systolic, early diastolic and end diastolic velocities, and peak tricuspid annular systolic, early diastolic and end diastolic velocities were determined from three consecutive cardiac cycles for patients with sinus rhythm, and from 10 consecutive cardiac cycles for patients with atrial fibrillation. Means were then calculated. Global left ventricular TDI peak systolic velocity (LVs) was defined as the mean value of the peak septal and lateral systolic velocities. The isovolumic contraction wave was determined as the wave preceding the systolic wave that begins before the peak of the R wave on the electrocardiogram (ECG). Myocardial acceleration during isovolumic contraction was measured by dividing the peak velocity (IVV) by the time interval from onset of the wave (zero-crossing) during isovolumic contraction to the time at the peak velocity of this wave as previously described (Figure 1).15 The interobserver and intraobserver variabilities for IVA measurements were 4.4% and 3.4% respectively. Right ventricular echocardiographic recovery (RVs, IVA, and IVV recovery) at sixth months after surgery was defined as the achievement of preoperative echocardiographic readings or improved echocardiographic measurements compared to preoperative echocardiographic findings.
Figure 1. Calculation of RVs and right ventricular IVA from tricuspid annular velocity curves obtained from the TDI studies (digitalized images).
Statistical Analysis
Statistical analysis was performed using SPSS software for Windows v.13.0 (SPSS Inc., Chicago, Illinois, USA). Data are presented as median values. The normality of data distribution was checked using the Kolmogorov-Smirnov test. Comparisons of data were performed using the non-parametric Mann-Whitney U and Wilcoxon tests. Pearson correlation coefficients were determined for continuous variables; the relationships between categorical variables were investigated by Spearman correlation analysis. Repeated measurements were compared by the Friedman test with Bonferroni correction; differences between any two measurements were compared using the Wilcoxon test. Comparison of more than two groups was performed using the Kruskal-Wallis test with Bonferroni correction; differences between any two groups were compared using the Mann-Whitney U test. Categorical data between 2 groups were compared by the Pearson c2 test. Logistic regression analysis was performed to determine predictors of functional recovery. In this model, pre-operative echocardiographic variables and NT proBNP levels were included as independent variables. In addition, differences between the preoperative and sixth month values for pulmonary artery pressure (Delta-PAP), left ventricular TDI peak systolic velocity (Delta-LVs), right ventricular echocardiographic recovery variables - including myocardial velocities of the lateral tricuspid annulus (Delta-RVs) and (Delta-RV IVA) - were evaluated in further logistic regression analysis. A probability of P<.05 was considered significant.
Intraobserver (the mean difference between two independent measurements) and interobserver (the mean difference between two independent observers) variabilities were analyzed in 20 randomly selected studies and expressed as the mean percentage error (difference divided by number of observations).
RESULTS
The study population initially included 80 patients. Seven patients died during the perioperative period and were excluded from analysis. Ten patients did not come for their first and/or sixth month follow-up appointments; these were excluded as well. The mean age of the remaining 63 patients was 49 (16) y (34 female and 29 male). Table 1 shows the clinical and echocardiographic characteristics of the study population. Forty patients were in sinus rhythm (63.5%) and 23 patients (36.5%) in atrial fibrillation at the time of surgery. The valve surgeries performed and their underlying pathologies were as follows: 16 patients (25.5%) with severe mitral regurgitation, 18 (28.5 %) patients with severe mitral stenosis, and 4 (6.5%) patients with combined mitral stenosis and regurgitation underwent mitral valve replacement (MVR) or repair surgery (RS); 8 (12.5%) patients with severe aortic regurgitation and nine (14.5%) with severe aortic stenosis underwent aortic valve replacement (AVR); and 8 (12.5%) patients with severe mitral and aortic valve disease underwent AVR and MVR. This group of patients represented 72% of all valvular surgeries performed within a 3 month period at our institution.
At the 6-month follow-up, the functional capacities of the patients had significantly improved (Preoperative NYHA Class II: 27 pts, Class III: 35 pts, Class IV: 1 pt; Postoperative 6 Month NYHA Class I: 20 pts, Class II: 36 pts, Class III: 7 pts; P<.026). After 6 months, 38 patients showed functional recovery. However, 25 patients did not show functional recovery compared to the preoperative period. Left atrial (LA), left ventricular end-systolic and left ventricular end-diastolic (LVEDD) sizes were significantly smaller than their corresponding preoperative measurements (median values for Wilcoxon test 4.14 cm vs 4.22 cm, P=.034; 3.28 cm vs 2.99 cm, P=.01; and 5.17 cm vs 4.88 cm, P=.008 respectively). A significant increase was seen in LVs (5.09 cm/s vs 7.12 cm/s; P<.001). Pulmonary arterial systolic pressures (PAP) were significantly improved as well (40 mmHg vs 32 mmHg; P<.001). No significant correlations between pulmonary artery systolic pressure and the values of the echocardiographic right ventricular systolic variables (RVs, RV IVA, RV IVV) were observed for the preoperative or postoperative sixth-month measurements.
Logistic regression analysis was performed to assess the independent predictors of functional recovery. Preoperative values of NT pro-BNP, LA, left ventricular ejection fraction (LVEF), PAP, LVs, RVs, RV IVA and perioperative pump duration were included in the model as independent variables. Analysis revealed that RV IVA (OR=3.1; 95% CI, 1.01-9.64; P=.047) and PAP (OR=1.07; 95% CI, 0.99-1.15; P=.07) were independent predictors of functional recovery at sixth months after surgery.
The effects of delta PAP, delta IVA , delta RVs and delta LVs on functional recovery were also evaluated via another logistic regression model. Delta PAP (OR=1.09; 95% CI, 1.02-1.16; P=.006) and delta IVA (OR=3.25; 95% CI, 1.27-8.33; P=.014) were found to be independent predictors of functional recovery at sixth months after surgery.
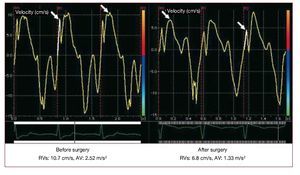
Early postoperative, postoperative first month, and postoperative sixth month RVs levels were significantly reduced (P<.001 for all comparisons). Tricuspid annulus-derived IVA and IVV values were reduced in the early postoperative period and at one month after surgery (early postoperative: IVA P<.001, IVV P<.001; 1 month after surgery: IVA P<.001 , IVV P<.001). However these figures had recovered by the sixth month after surgery; indeed, they were even higher than at baseline (IVA 2.45 m/s2 vs 2.62 m/s2; P=.014, IVV 8.60 cm/s vs 10.5 cm/s; P<.001) (Table 2). At 6 months of follow-up, 11 patients showed RVs recovery; 52 patients were still below the initial levels. Thirty-nine patients showed recovered RV IVA values, and 48 patients showed recovered RV IVV values. A strong positive correlation was seen between functional recovery and IVA recovery (Spearman's r=0.499; P<.001). No correlation was seen between RVs and RV IVV recovery. Thirty-one patients of the 38 patients who showed functional recovery experienced IVA recovery. Seventeen of the 25 who did show functional recovery also experienced no IVA recovery. (Pearson χ2 15.717; P<.001). No correlation was found between functional recovery and RVs and IVV recovery. Figure 2 shows one patient's preoperative RVs and RV IVA measurements and the same one month after surgery.
Figure 2. Demonstration of a patient's preoperative RVs and RV IVA measurements and the same at 1 month after surgery.
The patients were subgrouped according to the type of surgery they underwent (AVR, n=18; MVR or RS, n=37; AVR+MVR, n=8) in order to assess the impact of the type of procedure on their postoperative echocardiographic data. The preoperative echocardiographic data showed that the patients in the MVR or RS group had larger left atrial sizes (4.5 cm vs 3.9 cm; P=.007) and smaller left ventricular end diastolic sizes (5.1 cm vs 5.4 cm; P=.026) compared to those of the AVR group. The AVR+MVR group members had lower preoperative RVs than the MVR or RS group members. (8.9 cm/s vs 11.3 cm/s; P=.005). The AVR+MVR group members had larger left atrial sizes (4.5 cm vs 3.9 cm; P=.011) and smaller RVs values (8.9 cm/s vs 12.1 cm/s; P=.003) than those of the AVR group. Other preoperative echocardiographic data were statistically similar for all 3 subgroups.
The AVR, MVR or RS, and AVR+MVR subgroups were further analyzed according to their RVs values for the assessment of right ventricular function. The RVs values decreased in the early postoperative period, with this reduction persisting at sixth months of follow-up. The IVA and IVV levels decreased during the early postoperative period and were lower at one month post surgery, but initial values were recovered by 6 months of follow-up (Table 3).
The patients who underwent mitral valve repair surgery (RS; n=11) were compared with MVR patients (n=26). The RVs values were higher in patients who underwent RS during the early postoperative period and at 1 month after surgery (early postoperative: RS 6.18 cm/s vs MVR 5.03 cm/s, P=.027; one month after surgery: RS 7.14 cm/s vs MVR 5.48 cm/s; P=.007). By 6 months this difference had disappeared (RS 10.5 cm/s vs MVR 9.6 cm/s; P =.118). However, the IVA values were similar during the early postoperative period, at 1 month after surgery, and at 6 months of follow-up (RS 1.4 m/s2 vs MVR 1.2 m/s2; P=.337; RS 2.1 m/ s2 vs MVR 1.75 m/s2; P=.201; and RS 2.93 m/s2 vs MVR 2.49 m/s2; P=.056 respectively).
Patients with sinus rhythm on the day of surgery were compared to patients with atrial fibrillation. Preoperative echocardiographic assessment revealed that patients with sinus rhythm had smaller left atrial sizes (4.0 cm vs 4.8 cm; P=.003) and a lower pulmonary arterial systolic pressure (36.5 mmHg vs 46 mmHg; P=.026) than patients with atrial fibrillation. However, their RVs (sinus 11.5 cm/s vs AFib 11.1 cm/s; P=.096) and IVA (sinus 2.58 m/s2 vs AFib 2.29 V; P=.53) values were similar. During the postoperative follow-up the RVs values were similar (early postoperative: sinus 6.4 cm/s vs AFib 5.7 cm/s; P=.123; first month after surgery: sinus 6.7 cm/s vs AFib 5.7 cm/s; P=.078; 6 months after surgery: sinus 10.2 vs AFib 9.5 cm/s; P=.057). Similarly, the IVA values for the 2 groups were alike (early postoperative: sinus 1.08 m/s2 vs AFib 1.19 m/ s2; P=.575; one month after surgery: sinus 1.64 m/s2 vs AFib 1.89 m/s2; P=.372; 6 months after surgery: sinus 2.66 m/s2 vs AFib 2.69 m/s2; P=.977). The RVs values were lower at 6 months of follow-up in both groups compared to their preoperative values (sinus 11.5 cm/s vs 10.2 cm/s; P<.001; AFib 11.1 cm/s vs 9.5 cm/s; P<.001). The IVA values had recovered by sixth months of follow-up (sinus 2.58 m/s2 vs 2.66 m/ s2; P=.273; AFib 2.29 m/s2 vs 2.69 m/s2; P=.002).
Surgery was performed on 37 patients using antegrade blood cardioplegia, in 18 patients using retrograde blood cardioplegia, and in eight patients using combined antegrade and retrograde blood cardioplegia. No significant differences were seen between these groups with respect to functional and IVA recovery at six months of follow-up (Table 4). Neither was any significant correlation found between echocardiographic values and the aortic cross-clamp time, operation or pump duration, nor the maximum level of hypothermia.
DISCUSSION
Right ventricular dysfunction may occur during the postoperative period and is associated with increased mortality.22-25 Right ventricular dysfunction can persist up to one year in some patients after CABG.11,12 However, to our knowledge, no studies have investigated right ventricular function in patients undergoing isolated valve surgery. In this work the right ventricular functions of such patients were examined by TDI. One of the important findings of this study refers to the impairment in preoperative RVs values in the early postoperative period and the persistence of this impairment at one and sixth months after surgery. However, the novel and specific indicator of RV systolic function—the RV IVA value—recovered by 6 months of follow-up, a recovery that correlated with the functional recovery of the patients. Another important finding was that the preoperative RV IVA and PAP values (and their improvement over the postoperative period) were the only independent predictors of postoperative functional recovery; these provided more information than any other echocardiographic and biochemical variable.
Hedman et al11 reported the recovery of exercise capacity by 3 months after surgery, although right ventricular dysfunction persisted up to 1 year in their patients. Right ventricular IVA assessment seems to be a more sensitive technique than TDI-derived peak systolic velocity assessment in predicting contractility.16,17 Based on this information, the significant recovery in the right ventricular IVA values may help explain the improved exercise capacity observed. The results show RV IVA recovery to have occurred in patients with functional ecovery. This finding suggests that consecutive RV IVA measurements may predict the right ventricular function at six months of postoperative follow-up of patients who undergo isolated valve surgery. Perioperative deterioration in right ventricular function may delay or lead to the underestimatation of functional recovery after successful valve surgery. RV IVA assessment may therefore be useful in the clinical assessment of patients. Right ventricular dysfunction has been reported among almost all patients subjected to CABG independent of the cardiopulmonary bypass time, aortic clamp time or the cardioplegia technique used.1-4,22,23 Additionally, off-pump cardiac surgery does not seem to protect the right ventricle any better than on-pump coronary arterial bypass surgery.26 Right ventricular dysfunction seems to be associated with cardiac surgery per se, since no correlation was seen between the aortic cross-clamp time, pump time, the maximum hypothermia level or the type of cardioplegia protection in the present patients who had no coronary artery disease. Further, the right ventricular dysfunction rates were similar among the MVR, AVR and MVR+AVR subgroups. This finding supports the hypothesis that right ventricular dysfunction can develop independently from the underlying valvular pathology. However, the recovery of the right ventricular IVA values by 6 months after valvular surgery may help explain the significant functional improvement seen during the postoperative period. Although there was a persistent reduction in RVs values, the right ventricular IVA values obtained at the six month echocardiographic assessment correlated with functional recovery. Right ventricular IVA assessment may be useful and reliable in the postoperative follow-up of right ventricular function in patients regardless of their atrial rhythm.
Study Limitations
The use of any specific variable for the study of right ventricular systolic function after surgery suffers from the lack of a gold standard marker that allows appropriate comparisons to be made. In addition, myocardial velocity measurements with color-coded TDI methods reflect active contractions as well as passive myocardial movements. Hence, the re-analysis of our hypothesis with more specific methods such as myocardial strain and strain rate might be more reliable than using conventional tissue Doppler variables. Studies with larger numbers of patients and novel echocardiographic modalities would be useful for determining the association between functional recovery and right ventricular systolic function.
CONCLUSIONS
The present work shows that preoperative tissue Doppler-derived RV IVA and the pulmonary artery systolic pressure are independent predictors of 6-month functional recovery following isolated valve surgery. The improvement of these variables during the postoperative period, and their relationship with functional recovery, may be useful in the clinical assessment of patients following heart valve surgery.
ACKNOWLEDGMENTS
We would like to thank to Ferit Un (Statistics and Reporting Manager, Plato Statistics Medical Research, Istanbul, Turkey) for the effort invested in the statistical analysis of our database. We also thank Dr. Kenneth Nugent (Texas Tech University Health Sciences Center, Lubbock, TX) for his assistance in English during the preparation of this manuscript.
ABBREVIATIONS
AVR: aortic valve replacement
IVA: myocardial acceleration during isovolumic contraction
IVV: myocardial velocity during isovolumic contraction
LVs: global left ventricular peak systolic velocity on tissue Doppler imaging
MVR: mitral valve replacement
PAP: pulmonary artery systolic pressure
RVs: tricuspid annulus peak systolic velocity
on tissue Doppler imaging
TDI: tissue Doppler imaging
Correspondence: Dr C. Cevik.
Texas Tech University Health Sciences Center, Department of Internal Medicine,
3601 4th Street, 79430, Lubbock, TX, United States
E-mail: cihan.cevik@ttuhsc.edu
Received April 21, 2009.
Accepted for publication December 2, 2009.