Keywords
INTRODUCTION
In 2002, Cribier et al1 implanted the first transcatheter valve in the aortic position for the treatment of symptomatic severe aortic stenosis. The procedure was performed successfully in a patient with several co-morbidities and in cardiogenic shock who had been refused for surgical aortic valve replacement by several cardiac surgical teams. The technology experienced a rapid expansion in the years following this remarkable pioneering experience, and today >5000 transcatheter valves have been implanted worldwide. Importantly, the technology has experienced a prodigious development despite being restricted exclusively to patients considered at prohibitive or very high surgical risk. Thus, most patients who have undergone transcatheter aortic valve implantation (TAVI) to date have been octogenarians, with a predicted surgical mortality of >20% by logistic EuroSCORE or >8% by the Society of Thoracic Surgeons (STS) score. In addition, patients in the lower surgical risk score range (ie, <20% by logistic EuroSCORE or <8% by STS score) presented other major co-morbidities such as extreme frailty or porcelain aorta, which are not included in the surgical risk score calculators. Unlike other transcatheter techniques, transcatheter valve technology has evolved in an extremely high-risk population and this should always be taken into consideration when evaluating the results associated with TAVI procedures. In this article we review the main procedural aspects, acute and midterm clinical and hemodynamic results and potential complications associated with TAVI, and discuss future prospects in this field. For this purpose a literature search using PubMed, EMBASE, Cochrane Library, and Internet-based sources of information on clinical trials (www.clinicaltrials.gov, www.tctmd.com, www.cardiosource.com, www. theheart.org) was performed from November 2002 to September 2009 using "transcatheter/percutaneous/ transfemoral/transapical aortic valve implantation/ replacement/insertion" as subject headings.
TRANSCATHETER AORTIC VALVE IMPLANTATION: THE PROCEDURE
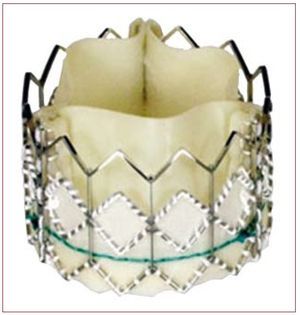
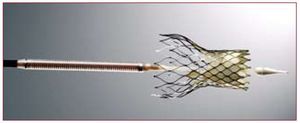
Two transcatheter aortic valves have been used in clinical practice in the last few years: the Edwards valve (Cribier-Edwards, Edwards SAPIEN, and SAPIEN XT; Edwards Lifesciences, Irvine, USA) and the CoreValve Revalving system (CoreValve, Paris, France; and Medtronic, Minneapolis, USA since February 2009). The Edwards SAPIEN valve consists of a trileaflet pericardial bovine valve mounted (sutured) in a stainless steel stent (or cobalt-chromium in the case of the SAPIEN XT) that is deployed by a balloon-expandable mechanism (Figure 1). The valve is available in 23-mm and 26-mm sizes. The procedures are performed in a catheterization laboratory or hybrid operating room, under general anesthesia and without cardiopulmonary bypass. In most cases the femoral artery is surgically exposed at the beginning of the procedure and sutured at the end of the procedure, although percutaneous closure devices are increasingly used. Balloon aortic valvuloplasty is systematically performed before valve implantation. The balloon-mounted valve is advanced through a 22F (23-mm valve) or 24F (26-mm valve) sheath with the Retroflex delivery catheter. After crossing the native aortic valve, the new valve is positioned using fluoroscopic, angiographic and transesophageal echocardiographic guidance and subsequently expanded under rapid pacing (180-220 beats/min) to minimize transvalvular flow and the risk of valve embolization. Litchenstein et al2 first described the transapical approach as an alternative to the transfemoral approach in those patients with non-appropriate (ie, too small, diseased or severely calcified) iliofemoral arteries. The transapical approach consists of directly puncturing the ventricular apex through a small left lateral thoracotomy and then advancing a 26F catheter through the ventricular apex up to the midventricular cavity. After crossing the aortic valve with a guidewire, the rest of the procedure follows the same steps in valve preparation and deployment as the retrograde transfemoral approach. It has been shown that the use of this double approach strategy makes it possible to treat a high proportion of elderly patients refused for standard surgical aortic valve replacement (SAVR).3,4 Interestingly, reports from centers using the 2 (transfemoral and transapical) approaches showed that from 32% to 52% of the patients were treated by the transapical approach due to the characteristics of the population, with a high prevalence of small or diseased iliofemoral arteries, and to the size of the catheters.3-5 Reducing the transfemoral catheter size to <20F (SAPIEN XT, 18F for the 23-mm valve and 19F for the 26-mm valve) in the near future will probably limit the use of the transapical approach to <30% of the patients. The CoreValve aortic valve consists of a trileaflet porcine pericardium valve mounted in a self-expanding nitinol stent (Figure 2). The valve is available in 26-mm and 29-mm sizes, and is implanted transfemorally using 18F catheters, which allows the percutaneous closure of the femoral artery without surgical cut-down in a high proportion of patients. Following balloon valvuloplasty, the valve is advanced across the native aortic valve with a delivery catheter and the self-expanding valve is deployed by retracting the outer sheath of the delivery catheter without the need of rapid pacing. In addition, the use of the subclavian approach as an alternative to the femoral approach appears promising for those patients with either small or diseased iliofemoral arteries.6 In this approach, a surgical cut-down is performed to isolate the subclavian artery just below the subclavian bone. Also, the first-in-human CoreValve implantation by transapical approach has recently been reported.7
Figure 1. Edwards SAPIEN valve.
Figure 2. CoreValve Revalving System.
PROCEDURAL RESULTS: PROCEDURAL SUCCESS AND 30-DAY MORTALITY RATES
First-in-Human and Feasibility Experiences
The main procedural results of the initial series including transfemoral and transapical approaches are shown in Table 1. Experience with the Edwards valve started in 2002,1 and the first single-center "first-in-human" registry was led by Dr Cribier in the Charles Nicolle hospital, Rouen, France.8,16 After this first-in-human experience, Dr Webb reported the initial experience of St. Paul's hospital in Vancouver, Canada, using the retrograde transfemoral approach.9,17 Two multicenter registries, the REVIVAL II in the USA (Transcatheter Endovascular Implantation of Valves; data presented at the Transcatheter Cardiovascular Therapeutics meeting 2006) and the REVIVE II in Europe (Registry of Endovascular Implantation of Valves in Europe II; data presented at the Transcatheter Cardiovascular Therapeutics meeting 2008) completed this initial experience with the Edwards valve/transfemoral approach. In 2006, Lichtenstein et al2 described the first 7 cases using the transapical approach, and Whalter et al10 reported the initial experience of the Leipzig University Heart Center, Leipzig, Germany, including 30 patients. Two published multicenter registries using the transapical approach in Europe11 and the USA,12 and the unpublished TRAVERCE (Transapical Surgical Delivery of the Cribier-Edwards Aortic Bioprothesis Clinical Feasibility) registry (data presented at the Transcatheter Cardiovascular Therapeutics 2008) completed the feasibility experience with the transapical approach. We were the first group to report the feasibility and initial results of a global program for the treatment of severe aortic stenosis including both the transfemoral and transapical approaches.3 Patients diagnosed with symptomatic severe aortic stenosis and refused for SAVR were evaluated by a team of interventional cardiologists and cardiac surgeons, and underwent transfemoral or transapical TAVI depending on aortoiliofemoral anatomy as determined by angiography and computed tomography. This selection process introduces a bias that, according to our understanding, precludes any comparison between the 2 approaches. In fact, patients treated by the transapical approach have systematically been associated with a higher risk profile than those undergoing the procedure by the transfemoral approach.4,5 In 2005, Grube et al18 reported the first implantation of a CoreValve Revalving system, and in 2006 the same group reported a first-in-human registry including a total of 55 patients.13 Following this first-in-human experience, a small single-center Canadian experience14 and a multicenter registry (Germany and Canada)15 were completed.
García et al19 and Moreno et al20 recently reported the first TAVI experience (n=4 in both studies) in Spain.
Recent Registries
Procedural and 30-day results of the largest recent multicenter registries of TAVI are shown in Table 2. The PARTNER EU (Placement of Aortic Transcatheter Valve European Union) registry included 130 patients from 9 centers in Europe who underwent TAVI (transfemoral and transapical approach) with the Edwards SAPIEN valve between April 2007 and January 2008 (data presented at the EuroPCR meeting 2009). This registry was followed by the SOURCE (Edwards Sapien Aortic Bioprothesis European Outcome) registry (data presented at the EuroPCR meeting 2009), which included a total of 1038 patients from 34 European centers who underwent TAVI using the Edwards SAPIEN valve. In total, 55% of the procedures were performed by transapical approach and 45% by transfemoral approach. This registry was begun as soon as the CE mark for the Edwards SAPIEN valve was obtained (September 2007). In addition, following CE mark approval, there was a registry including 646 patients who underwent TAVI with the CoreValve Revalving system in Europe.21 More recently, Laborde et al reported the results of a large European registry including a total of 1243 patients treated with the 18F CoreValve (data presented at the Transcatheter Cardiovascular Therapeutics meeting 2008).
Overall, the >90% procedural success rate and the <10% mortality rate obtained in most of these recent registries including >2000 patients provide further evidence of the safety and efficacy of this procedure as an alternative to SAVR in patients considered to be at prohibitive or very high surgical risk.
Transcatheter Aortic Valve Implantation: Predictive Factors of Procedural Success
Transcatheter aortic valve implantation is a challenging procedure that has up to now been applied only in a very high risk population. Furthermore, the technology has evolved very rapidly in the last 5 years, leading to lower profile and better valve delivery systems. Several groups have shown the importance of the learning curve by comparing their initial results with those obtained later on. Webb et al5 reported their experience with 168 patients treated with the Cribier-Edwards and Edwards SAPIEN valve implanted by transfemoral (n=113) or transapical (n=55) approach. The 30-day mortality rate decreased from 14.3% among the first 84 patients to 8.3% in the second half of the series (Figure 3). Himbert et al4 also reported that the early experience was the most important factor associated with in-hospital and mid-term mortality following TAVI. In addtion, Webb et al22 have recently shown that both an increase in experience and improvements in catheter/device design were associated with outstanding results, such as a 100% successful valve implantation rate and no mortality at 30 days. Similarly, Grube et al23 showed a 73% reduction in the 30-day mortality rate (from 40% to 10.8%) in a series of 102 patients treated with the 18F CoreValve Revalving system compared to the initial series of 10 patients treated with the first generation of 25F CoreValve. Both Edwards Lifesciences and CoreValve have established a training process for centers just starting out with TAVI procedures that consists of a 1-day to 2-day training course in a highly experienced center and then proctoring the first procedures with experienced physicians. There is no doubt that such a training process, by minimizing the mistakes associated with the learning curve, has made a major contribution to improving the results of TAVI. In addition, TAVI is a complex procedure that should be done in centers with high experience in percutaneous and cardiac surgery techniques by a multidisciplinary team of interventional cardiologists, cardiac surgeons, echocardiographists, anesthesiologists and internists/geriatricians. The involvement of this multidisciplinary team in patient selection, procedural and post-procedural time, and patient follow-up is an important factor for the success of a TAVI program.
Figure 3. Procedural success and 30-day mortality combined and separated by procedure type and experience. Combined (transfemoral and transapical approach) and transfemoral/transapical procedures are displayed by temporal halves. Reproduced from Webb et al5, with permission of the author and publisher.
Rodés-Cabau et al24 recently evaluated the prognostic factors of 30-day outcomes in the Canadian multicenter experience with the Edwards valve including a total of 345 TAVI procedures. The predictive factors of 30-day mortality were a history of pulmonary hypertension, severe mitral regurgitation, and the need for peri-procedural hemodynamic support. Interestingly, Buellesfeld et al25 found that a worse pre-procedural functional status as evaluated by the Karnofsky index was the only independent predictor of in-hospital mortality following TAVI with the CoreValve system, further highlighting the importance of patient selection on the results of this procedure.
MAJOR PERI-PROCEDURAL COMPLICATIONS
The type and incidence of the main peri-procedural complications associated with TAVI procedures are shown in Table 3.
Valve Malposition and Embolization
The incidence of valve malposition or embolization has decreased markedly from the initial first-in-human and feasibility series (around 6%) compared to the most recent studies (around 2%).8-25 This clearly reflects the importance of a learning curve on the process of valve positioning and implantation. However, the fact that the 2 valves currently available are not repositionable once fully deployed probably precludes reaching a 0% rate regarding this complication. The Edwards SAPIEN valve is deployed by balloon inflation under rapid pacing with little or no chance of repositioning during deployment. Fluoroscopy and angiography remain the gold standard for valve positioning and deployment, but in our experience the use of echocardiography plays an important role in improving valve positioning, especially in cases where the transapical approach is used.3,29 On the other hand, the CoreValve Revalving system is still repositionable when the valve is only partially deployed. However, no differences have been observed between the Edwards valve and the CoreValve regarding this complication.8-25 Although valve malpositioning and embolization can be successfully managed in most cases, future research should address this problem by developing fully repositionable transcatheter valves.
Need for Hemodynamic Support: Conversion to Open Heart Surgery
Transcatheter aortic valve implantation procedures can be associated with severe hemodynamic deterioration needing hemodynamic support (femoral-femoral bypass or counterpulsation balloon), due in part to both the type and severity of the disease and the high risk profile of the population being treated. However, this life-threatening complication and the need for emergent open heart surgery has decreased in recent series compared to initial experiences (from >3% to <2%),8-25 again highlighting the importance of the learning curve process in avoiding and managing procedural complications associated with these procedures. The time when some of the TAVIs were systematically performed on cardiopulmonary bypass circulation10,13 has passed, but we think that having an extracorporeal circulation machine available and a surgical team ready to put the patient on femoral-femoral cardiopulmonary bypass within a few minutes is still recommended. In fact, in the Canadian experience,24 the survival rate is 64% for patients who need hemodynamic support with extracorporeal circulation or intraaortic balloon pump during the procedure.
Major Access Site Complications
The large size of the catheters used for transfemoral TAVI procedures (from 18F to 24F), in addition to the very advanced age of the patients currently treated with this technology, has led to a high incidence (>10% in most series) of major vascular complications.8-25 A careful evaluation of the size, tortuosity and calcification of the iliofemoral system (by angiography and computed tomography, or by intravascular ultrasound in borderline cases) seems to be a key factor in avoiding such vascular complications. The experience with the CoreValve Revalving system suggested that reducing the catheter size can have a major impact on the incidence of vascular complications (>20% incidence in initial series vs <5% in recent registries).13-15,21,23 In contrast, maintaining the catheter size ≥22F in TAVI procedures performed with the Edwards valve translated into a fairly stable rate of major vascular complications over time, despite the increasing experience of the centers performing these procedures.8,9,16,17 Importantly, being able to treat these complications or having some backup with experienced peripheral interventionists is a major factor for improving procedural results and reducing 30-day mortality. The SOURCE registry showed, for the first time, that vascular complications were not associated with a higher 30-day mortality rate. This suggests that experienced teams dealing with these complications can limit their impact on short-term mortality.
Transapical procedures have also been associated with life-threatening access site complications, such as ventricular tears and major bleeding during apical repair.3-5,10-12,24,26 We have recently reported the potential usefulness of rapid pacing during apical repair in order to minimize ventricular tears.30
Stroke
The occurrence of cerebrovascular events has been a major concern from the very beginning of the TAVI experience. However, if we exclude the very initial series of patients who underwent CoreValve-TAVI under cardiopulmonary bypass,13-15 the reported stroke rate has consistently been <5% in most series,8-25 which is probably lower than expected in a population of octogenarians undergoing aortic valve procedures. It must be borne in mind that the rate of cerebrovascular events associated with SAVR in elderly patients is higher than 5%,31,32 suggesting that the aortic clamp and extracorporeal circulation might be associated with a higher risk of stroke than the manipulation of large catheters in the aortic arch, ascending aorta, and aortic valve annulus. Interestingly, the transapical approach avoids the manipulation of large catheters in the aorta and some groups have prioritized this approach over the transfemoral approach in patients with a severely diseased ascending aorta/aortic arch. Some studies have also found a tendency to a lower rate of cerebrovascular events with the transapical approach.4 The potential advantage of this approach for the prevention of peri-procedural strokes should be further explored.
Myocardial Infarction and Coronary Obstruction
The reported incidence of myocardial infarction (MI) associated with TAVI is extremely variable from one series to another, ranging from 0.2% to 17.5%.8-25 One important limitation is the lack of a universal definition for MI following TAVI. Furthermore, most studies do not specify the definition that was used for post-procedural MI. In addition to an asymptomatic increase in cardiac enzymes or the appearance of new Q-waves on the ECG, there have been cases of symptomatic left main coronary ostia obstruction following TAVI.17,33,34 Currently, it is well known that these coronary obstructions are not due to the jailing of the coronary ostia by the stent containing the transcatheter valve, but rather to the displacement of a heavily calcified aortic leaflet towards the coronary ostia.35 Several groups have emphasized the importance of measuring the distance between the aortic annulus and the coronary ostia before a TAVI procedure, especially in those patients with a bulky calcified valve. However, no specific recommendations exist as yet on the cutoff distance between the aortic annulus and coronary ostia which should contraindicate a TAVI procedure.
Acute Kidney Injury. Need for Hemodialysis
Patients currently undergoing TAVI have a high prevalence of chronic kidney disease (CKD). In fact, trying to avoid potential deterioration of renal function in patients with CKD has become an important argument for choosing TAVI over SAVR in such cases. However, TAVI procedures involve the administration of contrast media, the systematic occurrence of short periods of extreme hypotension (rapid pacing, balloon valvuloplasty, valve deployment), and the manipulation of large catheters in the aorta of patients with a high prevalence of diffuse atherosclerosis with the risk of cholesterol embolization, all of which are potential risk factors for acute kidney injury (AKI). Aregger et al36 evaluated the occurrence of AKI in 54 patients who had undergone TAVI with either the CoreValve or the Edwards SAPIEN valve. Most patients (56%) had improved glomerular filtration rate after TAVI but the incidence of AKI was 28% with up to 7.4% of the patients requiring hemodialysis during index hospitalization. Bagur et al37 recently reported an incidence of AKI of 11.7% following TAVI with the Edwards valve, which was associated with a 4-fold increase in the risk of postoperative mortality. Interestingly, in those patients with pre-procedural chronic kidney disease, the incidence of AKI was lower compared to those patients who underwent SAVR (9.2% vs 25.9%, respectively; need for hemodialysis, 2.5% vs 8.7%, respectively).
Intraventricular Conduction Abnormalities. Need for Permanent Pacemaker
Several studies have shown that TAVI procedures are associated with a high percentage of new intraventricular conduction abnormalities following the procedure.26-28 The stent containing the transcatheter valve may create either a direct mechanical stress or some degree of inflammation extending to the left bundle branch. Interestingly, a lower position of the valve with respect to the aortic annulus was associated with a higher rate of intraventricular conduction abnormalities in 2 studies.26,27 The need for a permanent pacemaker following the procedure is highly variable among the studies and seems to be higher with the CoreValve ReValving system (>10% in most studies, up to 33% in some series) than with the Edwards SAPIEN valve (<7%).8-25 These differences might be explained by the different design of the 2 devices, with the CoreValve being a much longer device with >5 mm of the stent containing the valve entering the left ventricular outflow tract.27 Jilahihawi et al28 suggested that in cases of TAVI with the CoreValve, the presence of pre-procedural left bundle-branch block with left axis deviation, interventricular septal dimension >17 mm, or noncoronary cusp thickness >8 mm predicted the likelihood of permanent pacemaker requirement with 75% sensitivity and 100% specificity. Future studies should further determine the predictive factors for permanent pacemaker implantation following TAVI and establish preventive measures to avoid this complication.
HEMODYNAMIC PERFORMANCE OF TRANSCATHETER VALVES
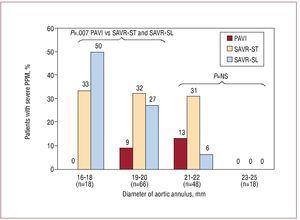
Unlike SAVR, which involves the removal of the native aortic valve prior to valve implantation, the mechanism of TAVI consists of the expansion of the stent containing the new valve against the calcified native aortic valve. Zegdi et al38 showed that the implantation of a percutaneous bioprosthesis within a "left-in-place" severely calcified valve might lead to incomplete or irregular expansion of the prosthetic valve. However, all TAVI studies with either the Edwards valve or the CoreValve have systematically shown very good hemodynamic results, with mean residual gradients following valve implantation of <15 mmHg and aortic valve areas >1.5 cm2, and these results have been maintained at mid-term follow-up.8-25 In a further step, we compared the hemodynamic performance of the Cribier-Edwards/Edwards SAPIEN valve (n=50) with that obtained in a matched population who had undergone SAVR with stented (Magna, Edwards Lifesciences) and stentless (Freestyle, Medtronic) valves.39 The study showed that TAVI provided superior hemodynamic performance compared to the surgical bioprostheses in terms of transprosthetic gradient and prevention of severe prosthesis-patient mismatch, especially in those patients with small (<20 mm) aortic annulus (Figure 4). Whereas valve sizing during SAVR is limited by the dimensions of the aortic annulus, TAVI is systematically performed with an oversized valve leading to some distension of the aortic annulus to accommodate the valve during balloon expansion. This is probably an important mechanism for obtaining better hemodynamic results. Moreover, although the TAVI valves are stented valves, the stent is much thinner than that of the stented valves used for SAVR and it therefore causes minimal obstruction to blood flow. However, TAVI has been associated with a high rate of paravalvular prosthetic regurgitation, with an incidence ranging from 65% to 85%, which is much higher than that observed following SAVR.39 The presence of the severely calcified native valve between the transcatheter implanted bioprosthesis and the aortic annulus probably precludes complete sealing of the paravalvular space and thereby leads to some degree of aortic regurgitation in most cases. Importantly, the vast majority of paravalvular leaks following TAVI are trivial or mild, with an incidence of moderate aortic regurgitation ranging from 0% to 26% and an incidence of severe aortic regurgitation ranging from 0% to 10%. The implantation of a second transcatheter valve has been shown to be an effective strategy for the treatment of moderate-to-severe transvalvular and paravalvular aortic regurgitation following TAVI.3,40 Finally, the degree of paravalvular aortic regurgitation generally remains stable or even improves slightly over time. Détaint et al41 suggested that a lower valve size/aortic annulus ratio was an important factor determining a higher degree of aortic regurgitation after TAVI. Future research efforts should focus on improving transcatheter valve technology to further reduce the occurrence of paravalvular leaks. In the meantime, given the uncertainty about the progression rate of the paravalvular leaks of the percutaneously implanted prosthetic valves in the long-term, these valves should be used with caution in patients with a long life expectancy.
Figure 4. Incidence of severe prosthesis-patient mismatch in the 3 aortic bioprosthesis groups (transcatheter valve, surgical stented valve, and surgical stentless valve) at 6-month to 1-year follow-up according to the aortic annulus size. PPM indicates prosthesis-patient mismatch; PAVI, percutaneous aortic valve implantation; SAVR-ST, surgical aortic valve replacement-stented valve; SAVR-SL, surgical aortic valve replacement-stentless valve. Modified from Clavel et al38, with permission of the author and publisher.
TRANSCATHETER AORTIC VALVE IMPLANTATION: MIDTERM RESULTS
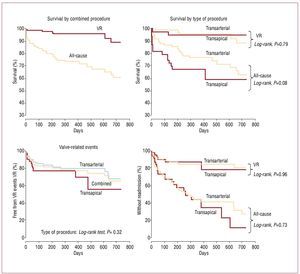
Few data exist on the mid-term results associated with TAVI procedures. Survival rates at 12-month follow-up associated with transfemoral TAVI procedures have increased from <80% in the initial series to ≥80% in the most recent series, such as the SOURCE registry.4,5,8,17,23,24 Interestingly, Webb et al5 recently showed that a high proportion of deaths during late follow-up are due to non-cardiac causes (Figure 5), highlighting the importance of patient selection. Transapical TAVI procedures have been associated with survival rates at 12-month follow-up of <80% even in recent series (SOURCE registry), probably due to the higher risk characteristics of the patients undergoing TAVI by transapical approach.12-14,24 The Canadian multicenter experience24 including transfemoral and transapical procedures showed that non-cardiac co-morbidities such as CKD and chronic pulmonary obstructive disease were 2 of the most important prognostic factors of worse late outcomes. Importantly, no structural failures of the transcatheter valves have been evidenced at mid-term follow-up.
Figure 5. Kaplan-Meier survival curves, valve-related mortality, free from valve-related (VR) events (death, infarction, stroke, reintervention) and valve-related readmission. Late survival was determined primarily by non-valve-related comorbidities. Reproduced from Webb et al5, with permission of the author and publisher.
TRANSCATHETER AORTIC VALVES: THE FUTURE
The Placement of Aortic Transcatheter Valve Trial
The PARTNER (Placement of Aortic Transcatheter Valve trial) trial is a prospective randomized multicenter (USA and Canada) trial including patients diagnosed with symptomatic severe aortic stenosis, divided into 2 cohorts: a) patients considered non-operable are randomized to transfemoral TAVI with the Edwards-SAPIEN valve versus medical treatment (primary endpoint: freedom from death at 1-year follow-up; superiority design); and b) patients at very high surgical risk are randomized to transfemoral or transapical TAVI versus SAVR (primary endpoint: freedom from death during study duration; non-inferiority design). The trial began in April 2007, the randomization period is expected to end by September 2009, and the first results are expected by the end of 2010. Feasibility studies and registries have shown promising results regarding TAVI. The PARTNER trial should provide definitive evidence for the treatment of patients with symptomatic aortic stenosis considered at prohibitive or very high surgical risk.
The Valve-in-Valve Concept
Bioprosthetic valves have limited durability, meaning that a large number of patients undergoing SAVR will need a reintervention in the future. There have been several reports of successful transcatheter valve implantation for the treatment of aortic bioprosthesis dysfunction,42-44 which opens a new avenue for the treatment of this challenging subset of patients. Importantly, the feasibility of this valve-in-valve strategy has been proven in stented and stentless aortic valve dysfunction. Future larger studies will have to determine the safety and efficacy of this valve-in-valve strategy.
Long-Term Outcomes
There are no data on the long-term performance of transcatheter valves. Obtaining these data in a prospective and scientifically rigorous way will provide valuable information about the durability of these valves, and will determine whether or not this technology could potentially be applied to younger and lower risk patients with symptomatic severe aortic stenosis.
Transcatheter Valve Technology: Research and Development
Several new transcatheter valves are currently in preclinical work or have already been tested in first-in-human trials45 (AorTx, Hansen Medical; Direct Flow, Direct Flow Medical; Enable, ATS [3F]; Heart Leaflet, Heart Leaflet Technologies; JenaValve, JenaValve Technology; Lotus, Sadra Medical; Lutter, German Research Foundation; Paniagua, Endoluminal Technology Research; Perceval, Sorin Group; PercValve, Advanced Bioprosthetic Surfaces; ValveXchange, ValveXchange; Zegdi, Zegdi, Coremove). In most of these new valves, pericardium and nitinol are the valve and stent materials, respectively, and most of them are repositionable before release. In addition, the 2 valves with clinical data, the Edwards SAPIEN valve and the CoreValve, will continue to improve their profile and deliverability, increase the choice of valve sizes, and explore new approaches (eg, subclavian). All these improvements in device technology should decrease the risks and improve the results of TAVI procedures, contributing to the expansion of this promising technology to a larger group of patients with severe aortic stenosis. In the next few years there will also be increasing clinical and echocardiographic data on the mid-term to long-term follow-up of transcatheter valves that will be very important in the process of consolidation and expansion of these procedures. However, acute and long-term results similar to or better than those of SAVR will have to be shown before extending the TAVI procedure to a lower risk population.
ABBREVIATIONS
AKI: acute kidney injury
CKI: chronic kidney injury
MI: myocardial infarction
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
Disclosure: Dr Josep Rodés-Cabau is consultant for Edwards Lifesciences.
Correspondence: Dr J. Rodés-Cabau.
Quebec Heart and Lung Institute,
2725 chemin Ste-Foy, G1V 4G5 Quebec City, Quebec, Canada.
E-mail: josep.rodes@criucpq.ulaval.ca