Recent years have seen an increase in the number of patients with advanced heart failure (HF).1 Accordingly, the following types of patients are increasingly being encountered: a) with advanced inotrope-dependent HF who not candidates for durable ventricular assist devices (dVADs) and who require inotropic support until they can undergo heart transplantation (HT); b) with group 2 pulmonary hypertension (PH) who are contraindicated for HT and are not candidates for dVAD therapy; and c) with the need for palliative care. For these situations, physicians in the United States use ambulatory intravenous milrinone perfusion, a modality practically unknown in Spain.
We analyzed all patients administered ambulatory milrinone perfusion between October 2015 and June 2022 in a transplant center. The study was approved by the ethics committee of the center. Informed consent was not considered necessary due to the retrospective observational nature of the study. In all patients, perfusion was initiated during hospital admission in conjunction with electrocardiographic monitoring to confirm the absence of severe adverse effects. All patients had to have been implanted with an implantable cardioverter-defibrillator (ICD), except those in palliative care. The continuous ambulatory perfusion was performed via a peripherally inserted central venous catheter using a portable CADD Legacy pump (ICU Medical, United States). Infusion speed was calculated for an approximate duration of 24hours and a dose of 0.3 to 0.4μg/kg/min. Patients and their family members were instructed on how to refill the pump and to dress the catheter at home. The patients attended the day hospital every 3 weeks for a clinical check-up and nursing care.
To analyze the usefulness of the treatment, we distinguished 3 groups with the following distinct objectives: a) as bridge to HT in inotrope-dependent patients (with success in this case considered HT without need to transition to urgent status); b) as bridge to candidacy in patients with PH who were contraindicated for HT (with success in this group considered achievement of a PH improvement permitting inclusion of the patient in the HT list); and c) as palliative care (in this case, the objective was to allow patients to remain at home with adequate symptom control until death). Overall, 19 patients were analyzed; their median age was 58 [53-67] years and 4 were women (21%).
Treatment objective was bridge to HT in 9 patients (47%), bridge to candidacy in 3 (16%), and palliative care in 7 (37%). The median treatment duration was 83 [35-229] days.
In the bridge to HT group, 7 patients (78%) achieved this objective as an elective procedure. Only 1 patient experienced primary graft failure, dying 7 days after the transplantation. The remaining patients survived more than 1 year after the HT. Two patients required implantation of a short-term ventricular assist device before the HT.
The 3 patients in the bridge to candidacy group due to PH were not candidates for dVAD due to biventricular HF. During the milrinone therapy, the median values of the mean pulmonary pressures fell from 46 [40-58] to 35 [33-37] mmHg while those of the median pulmonary vascular resistance fell from 5.8 [4.0-8.9] to 3.4 [2.5-4.5] WU. All patients used other oral pulmonary vasodilators, such as tadalafil and macitentan, and all patients achieved transplantation criteria (2 of them have since undergone transplantation, without primary graft failure and with successful outcomes).
Of the 7 patients treated with palliative intent, 4 (57%) remained at home with adequate symptom control until death. The other 3 experienced complications requiring treatment withdrawal (table 1).
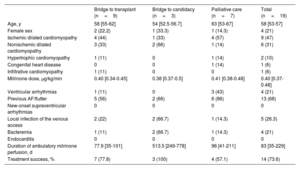
Characteristics of the patients who received ambulatory milrinone perfusion
| Bridge to transplant (n=9) | Bridge to candidacy (n=3) | Palliative care (n=7) | Total (n=19) | |
|---|---|---|---|---|
| Age, y | 58 [55-62] | 54 [52.5-56.7] | 63 [53-67] | 58 [53-57] |
| Female sex | 2 (22.2) | 1 (33.3) | 1 (14.3) | 4 (21) |
| Ischemic dilated cardiomyopathy | 4 (44) | 1 (33) | 4 (57) | 9 (47) |
| Nonischemic dilated cardiomyopathy | 3 (33) | 2 (66) | 1 (14) | 6 (31) |
| Hypertrophic cardiomyopathy | 1 (11) | 0 | 1 (14) | 2 (10) |
| Congenital heart disease | 0 | 0 | 1 (14) | 1 (6) |
| Infiltrative cardiomyopathy | 1 (11) | 0 | 0 | 1 (6) |
| Milrinone dose, μg/kg/min | 0.40 [0.34-0.45] | 0.38 [0.37-0.5] | 0.41 [0.38-0.48] | 0.40 [0.37-0.48] |
| Ventricular arrhythmias | 1 (11) | 0 | 3 (43) | 4 (21) |
| Previous AF/flutter | 5 (56) | 2 (66) | 6 (86) | 13 (68) |
| New-onset supraventricular arrhythmias | 0 | 0 | 0 | 0 |
| Local infection of the venous access | 2 (22) | 2 (66.7) | 1 (14.3) | 5 (26.3) |
| Bacteremia | 1 (11) | 2 (66.7) | 1 (14.3) | 4 (21) |
| Endocarditis | 0 | 0 | 0 | 0 |
| Duration of ambulatory milrinone perfusion, d | 77.9 [35-101] | 513.5 [249-778] | 96 [41-211] | 83 [35-229] |
| Treatment success, % | 7 (77.8) | 3 (100) | 4 (57.1) | 14 (73.6) |
AF, atrial fibrillation.
Results are presented as number of patients (%) or median [interquartile range].
Overall, ambulatory milrinone therapy achieved the desired objective in 74% of patients. The most frequent complication was local infection of the venous access, affecting 5 patients (26%). All patients responded satisfactorily to targeted antibiotic therapy. No case of endocarditis was recorded. Four patients (19%) developed sustained ventricular tachycardia (SVT) during the treatment; it was successfully treated with an ICD discharge but required milrinone withdrawal in all cases. SVT was most frequent in the palliative group (3 of the 4 cases) and in patients not treated with beta-blockers (8% vs 60%; P=.053). No other adverse effects requiring treatment cessation were recorded. There were no deaths directly attributable to milrinone.
Ambulatory milrinone therapy is barely used in Spain. The present series is the first to report the experience in Spain with this therapy. Although no large randomized studies have been conducted with ambulatory milrinone, some series have been published showing that milrinone can be useful in the above-mentioned contexts:
- •
As palliative treatment: the available registries show improved quality of life2-4; this is in line with our experience, with hospitalizations and emergency department visits avoided in 57% of patients.
- •
As bridge to HT: the published registries report success rates for this strategy of 65% to 92%,3,4 similar to that of our series (78%).
The most frequent complications are catheter infections; however, these are usually local and have little impact on patients’ quality of life. The most severe complication is SVT, which is more common in palliative care and in patients not receiving beta-blocker therapy; however, ICD therapy correctly treated all cases of SVT in our series.
In conclusion, we present the first experience in Spain with ambulatory milrinone therapy, which could be an alternative treatment option in patients with advanced HF as bridge to HT, bridge to candidacy, or palliative care. In our series, the success rate of this therapy was 74% and its safety profile was acceptable for the patients’ characteristics.
AUTHORS’ CONTRIBUTIONSJ.M. Viéitez Flórez and F.J. Hernández Pérez contributed to patient care, data collection, and manuscript drafting. C. Mitroi, S. Jiménez Lozano, M. Gómez Bueno, and J. Segovia Cubero contributed to patient care, data collection, and manuscript revision.
FUNDINGNone.
CONFLICTS OF INTERESTNone.
We thank Dr Mercedes Rivas Lasarte for correcting the article and helping with the statistical analysis and for her connection to this project.