Transcatheter aortic valve implantation (TAVI) has become a highly safe procedure, and its indication has been extended to intermediate- and low-risk patients in the latest clinical practice guidelines.1 This exponential spread of TAVI procedures can be at least partially explained by the continuous technical updates of prostheses and delivery systems. However, most prostheses still require preservation in glutaraldehyde to ensure the integrity of the leaflets, as well as a crimping process in the catheterization laboratory, which requires specifically trained staff and may prolong procedural times.2 The new Vienna self-expandable prosthesis (P&F, Germany) explores the novel option of a precrimped prosthesis with dehydrated bovine pericardium that can be rapidly rehydrated with saline solution immediately before implantation, facilitating storage, shortening implant times, and potentially reducing costs. The valve is composed of a nitinol stent (without radiopaque markers to guide positioning), an external polytetrafluoroethylene sealing skirt, and a supra-annular design for optimal hemodynamic performance. The delivery system allows a sheathless implant with an 18-Fr profile for all sizes, as it incorporates an inline sheath similarly to other self-expanding prostheses.
We present the first-in-human experience with the new-generation Vienna valve through the case of an 81-year-old woman with severe aortic stenosis in New York Heart Association functional class II, and a history of coronary artery disease and hypertension. Informed consent was obtained. Echocardiographic study showed a trileaflet aortic valve with a valve area of 0.8cm2 (0.47cm2/m2), peak and mean gradients of 68 and 44mmHg respectively, and a peak velocity of 4.2 m/s, without significant regurgitation and preserved ejection fraction (60% by Simpson biplane). Using computed tomography, a perimeter of 72.8mm (diameter derived from the 23.1-mm perimeter) was measured, with an Agatston score of 2214 (severe calcification). These dimensions corresponded to a 29-mm Vienna prosthesis and a degree of overexpansion of 21% with respect to the size of the ring. A high origin of the coronary arteries with respect to the valvular plane was confirmed (right coronary: 13.2mm, left coronary: 21.8mm), as well as adequate femoral vascular accesses (right: 9.5 x 8.6mm; left: 8.7 x 8.5mm) without significant calcification or vascular tortuosity. The patient had first-degree atrioventricular block, without other significant electrical disturbances, and a EuroSCORE II of 1.29%.
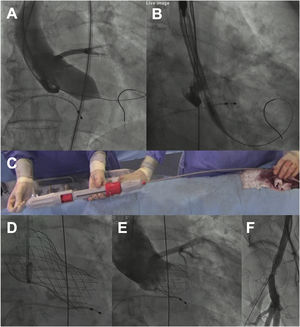
The procedure (video 1 of the supplementary data) was performed under mild sedation, through a right femoral access and preclosed with 2 Proglide devices (Abbott Vascular, USA). A high-support guidewire was positioned into the left ventricle and aortic balloon valvuloplasty was performed using a noncompliant 22-mm balloon under rapid pacing. Then, after briefly flushing the 3 ports of the system with saline and performing manual elongation of the delivery system to avoid gaps between the capsule and the rest of the system, it was advanced without an introducer (inline sheath). As a whole, the preparation time of the system was less than 1minute. For implantation, the distal end of the capsule was positioned at the level of the aortic annulus in the cusp overlap view and the valve was released with slow clockwise rotation of the knob located at the distal edge of the handle (figure 1A), allowing a controlled and precise positioning of the prosthesis at all times. In this phase, rapid pacing was not necessary. In addition, although recapture of the prosthesis was not necessary, it is an easy step that can be performed by simply making a knob counter-clockwise rotation maneuver. In our case, the prosthesis showed adequate radial strength, achieving complete expansion without residual paravalvular leaks or the need for postdilation (figure 1B). The patient had no rhythm disturbances or vascular complications.
A: balloon valvuloplasty with True Dilatation 22-mm balloon (Bard Vascular Inc, USA). B: positioning of the capsule in the aortic annulus. C: advancement of the delivery system with the Vienna precrimped prosthesis. D: Vienna valve positioned. E: follow-up aortogram without residual paravalvular leak. The positioning was high (about 3mm deep in 2-cusp view before final release) but ended up at 7mm depth in 3-cusp view, within the accepted range (3-8mm). F: follow-up angiogram of the right femoral artery without spontaneous contrast output.
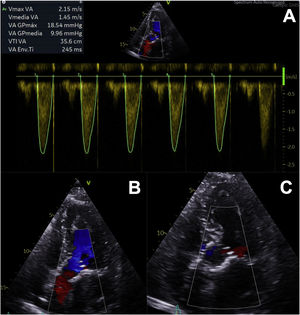
In the following 24hours, the patient developed complete atrioventricular block, requiring definitive dual-chamber pacemaker implantation. Echocardiographic follow-up study demonstrated adequate valve function, with a significant reduction in mean gradient (10mmHg), aortic valve area of 2cm2, and no periprosthetic leaks (figure 2), and consequently she could be discharged 72hours after implantation. There were no events during 1 month of follow-up.
Although a preliminary experience with a former iteration has been published,2 this is the first-in-man report with the current iteration of the Vienna valve, as part of the ongoing VIVA trial (NCT04861805) and is also the first percutaneous implantation of the Vienna aortic prosthesis performed in Spain. The innovative preassembled system avoids the need for specifically trained staff, simplifying the procedure, avoiding possible crimping mistakes and, therefore, potentially reducing both the time of the procedure and the related technical costs, which can be especially useful in emergent implants.2 Currently, it is available in 4 sizes (23, 26, 29, and 31mm), and has a user-friendly delivery system that allows controlled release of the platform and the possibility of repositioning and recapturing the device until 90% of the valve has been released.
Conduction disturbances continue to be the Achilles heel of most self-expanding valves.1 In this regard, although our patient developed complete atrioventricular block, it is still early to draw conclusions. The possibility of recapturing and repositioning the prosthesis could minimize the risk of electrical disturbances, perivalvular leaks, mitral valve involvement, and coronary occlusion.
The initial experience with the self-expanding Vienna valve in the VIVA study seeks to demonstrate the short-term safety and effectiveness in order to obtain CE mark approval. Its innovative precrimped system may reduce both procedural time (essential in emergent scenarios) and costs, while maintaining an adequate radial strength and navigability. Studies with longer-term follow-up will undoubtedly be necessary to determine the durability of leaflets treated with this particular method.2
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSDr Amat-Santos contributed to the study design. J.C. Gonzalez-Gutiérrez, S. Blasco-Turrión, A. Campo, and J.P. Sánchez-Luna collected and analyzed the information. All authors approved the final version of the manuscript.
CONFLICTS OF INTERESTThe institution participates in the VIVA trial and receives research grants from Products & Features. I.J. Amat-Santos is proctor for Products & Features.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.10.008