Over the years, there has been an increase in the number of patients on circulatory assist devices who require urgent heart transplantation (HTx).1
The short-term Impella CP left ventricular assist device (Abiomed; Danvers, Massachusetts, United States) is a continuous-flow axial pump placed across the aortic valve that drives the blood directly from the left ventricle toward the ascending aorta.2 Conventional implantation of the device is by femoral access through a 14-Fr catheter, and it provides a maximum theoretical flow rate of 4 L/min, although the rate in vivo does not usually exceed 3.5 L/min. In the product insert, the recommended duration of ventricular support is <7 days. The device has been successfully used in Spain as a bridge to HTx.3 According to data from the Spanish Transplant Organization Registry, 73% of patients categorized as HTx urgency 0 wait 10 days or less for transplantation.4 Nonetheless, time on the HTx waiting list is unpredictable in individual patients and has been seen to increase in the last few years.1 Use of the femoral access requires immobilizing the patient during the wait prior to HTx, making rehabilitation more difficult and increasing the risk of complications following the procedure.
Abiomed has designed a kit for transaxillary implantation of the Impella 2.5, Impella CP, and Impella 5.0 devices. Studies in humans support the use of Impella 5.0 and Impella CP for the treatment of cardiogenic shock,3 although the Impella 5.0 system requires larger caliber catheters (21 Fr), with a potential risk of vascular complications.
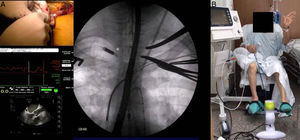
We report on 4 patients who received an Impella CP as a bridge to HTx using a transaxillary approach between March and December 2016, with follow-up to 31 May 2017. We analyzed survival, the duration of mechanical ventilation following HTx, length of ICU stay, and adverse events such as bleeding and infection. Impella CP implantation was carried out in the surgical theater using a right infraclavicular incision to expose the axillary artery. Following heparin administration, an end-to-side anastomosis of a Dacron graft (10mm) to the artery was performed. The device introducer was inserted through the graft and secured with the graft lock, and a rigid guidewire was advanced to the left ventricle. The device was then mounted on the guidewire (monorail system) and advanced to the correct position with the aid of fluoroscopy and transesophageal echocardiography guidance (Figure A).
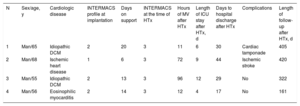
The patient characteristics and study variables are shown in Table. All patients were extubated following Impella CP implantation, and a prompt rehabilitation program was started (kinesiotherapy, physiotherapy, and active movements) during support with the device. The median time assistance was required was 13.5 [interquartile range, 11.25-15.5] days. Anticoagulation was performed with a solution of dextrose 5% and heparin at a concentration of 50 IU/mL. HTx was successfully carried out, and the 4 patients were discharged after a mean ICU stay of 7.5 [5.5-9.75] days and a median time to hospital discharge of 29.5 [26-33.5] days following HTx. The main postprocedure complications were 1 case of cardiac tamponade at 9 days following HTx and 1 case of ischemic stroke in a patient with significant bilateral carotid stenosis, who recovered without sequelae. After a median follow-up of 363 days, all patients were alive and in functional class I.
Patients Undergoing Impella CP Circulatory Assistance as a Bridge to Heart Transplantation
| N | Sex/age, y | Cardiologic disease | INTERMACS profile at implantation | Days on support | INTERMACS at the time of HTx | Hours of MV after HTx | Length of ICU stay after HTx, d | Days to hospital discharge after HTx | Complications | Length of follow-up after HTx, d |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Man/65 | Idiopathic DCM | 2 | 20 | 3 | 11 | 6 | 30 | Cardiac tamponade | 405 |
| 2 | Man/68 | Ischemic heart disease | 1 | 6 | 3 | 72 | 9 | 44 | Ischemic stroke | 420 |
| 3 | Man/55 | Idiopathic DCM | 2 | 13 | 3 | 96 | 12 | 29 | No | 322 |
| 4 | Man/56 | Eosinophilic myocarditis | 2 | 14 | 3 | 12 | 4 | 17 | No | 161 |
DCM, dilated cardiomyopathy; HTx, heart transplantation; ICU, intensive care unit; MV, mechanical ventilation.
To our knowledge, this is the first experience presented in Spain of Impella CP implantation using a transaxillary approach as a bridge to HTx. Although the mean duration of ventricular support was longer than recommended, there were no cases of device dysfunction and only 1 patient (No. 3) experienced hemolysis, evidenced by haptoglobin consumption on the tenth day of support, with no clinical repercussions. This event was resolved by adjusting the revolutions on the Impella CP. None of the devices had to be repositioned due to displacement, likely because of the shorter intravascular route, which may increase the stability of the device. Therefore, we consider the Impella CP short-term left ventricular assist device to be very useful for patients with predominantly left dysfunction in HTx urgency 0.
The transaxillary approach performed by experienced teams is safe and enables prompt mobilization and physiotherapy (Figure B). This contrasts with transfemoral implantation of the Impella CP or use of a peripheral extracorporeal membrane oxygenator, which oblige the patient to remain in bed, thereby favoring a loss of muscle mass, slowing functional recovery, and increasing the risk of complications.
Although our series is small, there were no complications directly related to the transaxillary approach. The case of cardiac tamponade occurred some days following HTx and was treated with pericardiocentesis. The patient with ischemic stroke showed no focal neurologic symptoms during Impella CP support and the event was interpreted as a complication of surgery in the context of significant carotid disease. Studies including larger numbers of patients will more clearly establish the usefulness of this strategy.