Keywords
INTRODUCTION
Since its early description in 1924, Libman-Sacks endocarditis has been considered the most characteristic cardiac disorder of systemic lupus erythematosus (SLE), although not the most frequent.
The frequency of endocardial disorder in SLE varies according to the diagnostic technique used, although clinical diagnosis is uncommon. In the experience of some authors, the lesions are clinically significant in just 20% of patients1 and usually progress slowly after they have had the disease for many years.1,2
In the few cases where surgical valve repair is necessary, bioprosthetic valves are not recommended, since these can lead to Libman-Sacks endocarditis3; however, in relation to this there is controversy in the medical literature.
We present a case of Libman-Sacks endocarditis involving the mitral valve with rapid progression to severe regurgitation treated with reparative surgery via mitral annuloplasty.
CLINICAL CASE
Woman, 36 years old, diagnosed with connective tissue disease, presenting incomplete clinical criteria for SLE for 8 years, small vessel vasculitis and repeated miscarriages, without previous cardiological symptoms.
Admitted for resting dyspnea and marked bloody sputum; at exploration she presented tachypnea, increased central venous pressure, bilateral rales up to the middle lung field, and grade IV/VI pansystolic mitral murmur. She also presented distal vasculitis lesions in the fingers and toes. Routine analysis revealed anemia with hemoglobin 9.3 g/dL. Repeated testing for antiphospholipid antibodies proved negative.
Electrocardiogram demonstrated sinus tachycardia without other associated anomalies. Chest x-ray at admission showed mild cardiomegaly and bilateral basilar interstitial lung pattern.
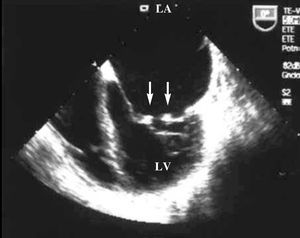
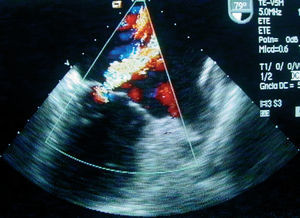
Transthoracic and transesophageal echocardiography showed mobile verrucose nodular thickenings of 2-3 mm in both mitral valves indicative of Libman-Sacks endocarditis (Figure 1), and severe mitral regurgitation (Figure 2). Systolic size and function of both ventricles were preserved.
Figure 1. Transesophageal echocardiogram: apical 4-chamber projection where small vegetations can be observed on both mitral valves (arrows). LA indicates left atrium; LV, left ventricle.
Figure 2. Transesophageal echocardiogram: 79-degree projection where a severe mitral regurgitation is visualized. LA indicates left atrium; LV, left ventricle.
Transthoracic echocardiography was done 2 years earlier. Nodular thickenings, a few millimeters in size, were seen in the mitral valve and mild mitral regurgitation compatible with Libman-Sacks endocarditis.
Given the suspicion of intraparenchymal pulmonary hemorrhage, high-resolution computerized tomography was requested that confirmed the diagnosis (images showing bilateral masses with ground glass opacity).
Evolution
Following high-dose corticosteroids and intensive diuretic treatment, hemoptysis was eliminated and the marked heart failure controlled. However, the patient remained in functional grade III due to which, 4 months after first admission, the valvular heart disease was treated via extracorporeal surgery and mitral valve repair (due to the high risk of bleeding in this patient) with a Carpentier ring.
Macroscopic visualization of the valve showed 2-3 mm granulomas and pathological anatomy showed fragments with fibrin deposits and nonspecific focal calcification, but compatible with the clinical diagnosis.
One year later, the patient remained free of cardiological symptoms; echocardiogram showed a mild residual mitral regurgitation similar to that at postsurgery discharge.
DISCUSSION
Libman-Sacks endocarditis is characterized by verrucose lesions on the valve surfaces, although they also can be found on the free margins of the valves and, in general, in any area of the atrial or ventricular endocardium; verruca histology is usually nonspecific.4
Doppler echocardiography can be considered the technique of choice for diagnosis, with an incidence of valve disease between 18% and 50%1,5,6 and up to 74% if transesophageal echocardiography is used.7
This incidence, however, is much higher than the number of patients with lupus who present clinically important lesions, representing around 20% of cases.1
Although the origin of valvular lesions in SLE is closely linked to the presence of antiphospholipid antibodies,4 negative test results, as in our case, are described in the medical literature in other patients with SLE and Libman-Sacks endocarditis8 or even in nonbacterial thrombotic endocarditis without underlying disease.9 This is probably explained by the fact that currently all the prothrombotic situations that can lead to the appearance of these lesions remain undefined.
In our patient, echocardiography showed small multiple mobile verrucose lesions in the mitral valve free margins, both in transthoracic and transesophageal echocardiography (Figure 1).
The most frequent functional disorder is regurgitation, as in this case. However, when the disease has progressed for only a few years, the patient is young and lupus is active, the valvular lesions are seldom sufficiently severe to induce heart failure.1,2,10
In our case, an echocardiographic study 2 years earlier, demonstrating Libman-Sacks vegetations and mild mitral regurgitation, makes it possible to demonstrate rapid progression to massive mitral regurgitation with congestive heart failure, due to which surgical intervention was indicated. There are barely fifty cases published in the medical literature on mitral valve repair due to SLE; in a prospective study it was demonstrated that 8% of the patients finally need heart surgery.1
The treatment of the patients with lupus-associated valvulopathy includes endocarditis prophylaxis, antiaggregant or anticoagulant treatment in selected cases, and valve replacement when the valvular disorder is severe11; the role of corticosteroid treatment regarding this valvulopathy remains undetermined.11 Controversy exists concerning the type of surgical intervention. Some authors suggest the superiority of mechanical prostheses in this type of disorder vs bioprotheses, including cryopreserved homografts, since the latter can lead to lupus valvulitis on the new valve.3,12-14 However, other authors15 have advocated reconstructive surgery to avoid the drawbacks of a mechanical prosthesis in young patients who normally need high doses of corticosteroids and anticoagulant therapy and who have a high incidence of kidney failure.
In our case, the 2 recent episodes of massive pulmonary hemorrhage with severe hemoptysis, and the need for high doses of corticosteroids to control the disease guided the choice of reparative surgery of the valve. Although the evolution of the patient will have to be monitored in the coming years via serial echocardiographic studies, 1 year after surgery the state of the valvular repair is optimal.
Correspondence: Dr. J. Fernández-Dueñas.
Plaza de Colón, 24 2.o dcha. 14001 Córdoba. España.
E-mail: jaimefdf@hotmail.com