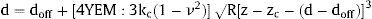We investigated the anatomical localization, biomechanical properties, and molecular phenotype of myocardial collagen tissue in 40 patients with severe aortic stenosis with preserved ejection fraction and symptoms of heart failure.
MethodsTwo transmural biopsies were taken from the left ventricular free wall. Mysial and nonmysial regions of the collagen network were analyzed. Myocardial collagen volume fraction (CVF) was measured by picrosirius red staining. Young's elastic modulus (YEM) was measured by atomic force microscopy in decellularized slices to assess stiffness. Collagen types I and III were measured as CIVF and CIIIVF, respectively, by confocal microscopy in areas with YEM evaluation.
ResultsCompared with controls, patients exhibited increased mysial and nonmysial CVF and nonmysial:mysial CVF ratio (P < .05). In patients, nonmysial CVF (r = 0.330; P = .046) and the nonmysial:mysial CVF ratio (r = 0.419; P = .012) were directly correlated with the ratio of maximal early transmitral flow velocity in diastole to early mitral annulus velocity in diastole. Both the CIVF:CIIIVF ratio and YEM were increased (P ≤ .001) in nonmysial regions compared with mysial regions in patients, with a direct correlation (r = 0.895; P < .001) between them.
ConclusionsThese findings suggest that, in patients with severe aortic stenosis with preserved ejection fraction and symptoms of heart failure, diastolic dysfunction is associated with increased nonmysial deposition of collagen, predominantly type I, resulting in increased extracellular matrix stiffness. Therefore, the characteristics of collagen tissue may contribute to diastolic dysfunction in these patients.
Keywords
Aortic valve stenosis (AS) is a common disease in which failure of the aortic valve to completely open imposes an abnormally high pressure load upon the left ventricle that, in turn, results in cardiomyocyte hypertrophy and myocardial fibrosis.1 Myocardial fibrosis has been reported to be associated with increased left ventricular (LV) stiffness, increased LV end-diastolic pressure, and impaired diastolic filling (ie, diastolic dysfunction).2–5 Progressive myocardial fibrosis that contributes to worsening of diastolic dysfunction may eventually facilitate the presentation of the signs and symptoms of heart failure (HF) in AS patients with preserved ejection fraction (PEF).2,3,6
In a pioneer study, Villari et al.7 demonstrated that an increase in the amount of collagen tissue per se does not alter LV diastolic function in patients with AS, suggesting that the impact of myocardial fibrosis on LV function is not just a matter of the quantity of collagen but also of its quality. We thus hypothesized that, in patients with severe AS with PEF and symptoms of HF, excessive myocardial collagen deposition and alterations in the anatomical localization, biomechanical properties and molecular phenotype of these deposits, may be associated with diastolic dysfunction. To test this hypothesis, we analyzed the localization, either mysial (ie, associated with groups of cardiomyocytes and surrounding and interconnecting individual cardiomyocytes) or nonmysial (ie, interstitial and perivascular) of collagen deposits, the stiffness of the decellularized tissue, and the relative abundance of collagen types I and III fibers in relation to stiffness in the myocardium of patients with severe AS with PEF and symptoms of HF who underwent surgical aortic valve replacement. In addition, the association of these aspects with LV filling pressures was analyzed.
METHODSStudy PopulationAll participants gave written informed consent to participate in the study, and the the study protocol was approved by the institutional review committee. The study conformed to the principles of the Helsinki Declaration.
Forty patients were recruited with stage D18 severe AS scheduled for surgical aortic valve replacement. All patients had a previous clinical diagnosis of chronic HF based on the presence of at least 1 major and 2 minor Framingham criteria.9 Patients presenting with angina or syncope but without evidence of previous HF were not included. All patients had at least 1 previous hospitalization for class IV dyspnea secondary to HF. All patients were in New York Heart Association functional classes II to IV and exhibited a LV ejection fraction ≥ 55%. Patients with significant coronary artery disease (≥ 70% stenosis in any epicardial coronary artery, and/or ≥ 50% in left main artery), previous acute coronary syndrome or revascularization procedure and stage 4-5 chronic kidney disease were excluded. Two transmural biopsies were taken in each patient from the LV free wall between the left descending and the circumflex coronary artery by means of a tru-cut needle during the aortic valve replacement procedure.
Cardiac samples were collected from 10 age- and sex-matched participants who had died of noncardiovascular-related diseases, and were processed for histomorphological study to be used as controls. None of them had a previous history of cardiovascular disease and evaluation of the autopsy files ascertained the absence of macroscopic and microscopic cardiac lesions.
Echocardiographic StudyStandard echocardiographic examinations were performed in all patients by 2 experienced operators less than 1 week before surgery, using an iE33 digital ultrasound system (Philips). Images were obtained on exhalation to account for the slight variations in blood flow while breathing. Average measurements from 3 cardiac cycles were obtained (5 in the case of atrial fibrillation). Neither significant mitral valve disease nor aortic regurgitation was present in patients. Thirty-five patients were in sinus rhythm and 5 in atrial fibrillation. Medication was not discontinued before the echo exam. Left ventricular and left atrial dimensions were measured according to the American Society of Echocardiography recommendations.10 Left ventricular mass was calculated using the Devereux formula and was indexed by body-surface area (LV mass index). Left ventricular hypertrophy was defined as a LV mass index greater than 115g/m2 in men and greater than 95g/m2 in women.10 According to this definition, all patients exhibited LV hypertrophy. LV ejection fraction was measured using the Simpson method. Diastolic function was evaluated by analysis of the maximum early (E) and late (A) transmitral flow velocities in diastole, pulmonary vein flow, mitral flow propagation velocity, and tissue Doppler indexes at the septal and lateral mitral annulus. The ratio E:maximum early diastolic velocity of the average of the septal and lateral mitral annulus displacement (e’) was calculated. The absence or presence of high LV filling pressures was established on the basis of E:e’ ratio values ≤ or > 15, respectively.11 Mean and maximal transvalvular pressure gradient and aortic valve area were assessed in all patients.
Myocardial BiopsyTwo transmural biopsies were taken from the LV free wall between the left descending and the circumflex coronary artery by means of a tru-cut needle during the aortic valve replacement procedure, before patients were placed on extracorporeal circulation. One specimen was fixed immediately in 4% formaldehyde and embedded in paraffin. In the last 20 patients, another specimen was frozen in optimum cutting temperature compound for subsequent atomic force microscopy measurements. No complications occurred during or after the biopsy procedure.
Biochemical DeterminationN-terminal pro-B-type natriuretic peptide was measured in plasma samples by an ELISA method (Roche Diagnostics).
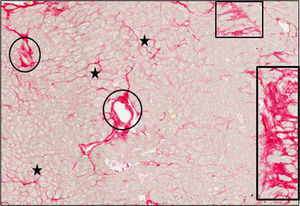
Histomorphological StudyThe fraction of myocardial volume occupied by collagen tissue (CVF) was determined by quantitative morphometry with an automated image analysis system in sections stained with collagen-specific picrosirius red (Sirius red F3BA in aqueous picric acid). The endocardium was excluded from the analysis. Two localizations for collagen deposition were defined: mysial (thin bands associated with groups of cells or perimysium and collagen which surrounds and interconnects individual cells or endomysium), and nonmysial (large strands localized in the interstitial and perivascular spaces) (Figure 1). The CVF measurements from 2 patients were discarded due to image artifacts caused by abnormal morphology of the processed myocardial tissue (excessive thickness of the endocardium and abnormally low subendocardial tissue) leading to aberrant picrosirius red staining (with percentages of CVF higher than 50%). In the 38 remaining patients with valid CVF assessments, total and nonmysial CVF were measured and mysial CVF was calculated as the subtraction of nonmysial CVF from total CVF.12 In addition, the nonmysial CVF:mysial CVF ratio was calculated in each patient.
Endomyocardial tissue from 1 patient with severe AS with PEF and symptoms of HF. Sections were stained with picrosirius red and collagen fibers were identified in red. Whereas mysial collagen was identified as thin bands surrounding individual cardiomyocytes or groups of cardiomyocytes (stars), nonmysial collagen was identified as large strands diffusely localized across the interstitium (rectangles) and around intramyocardial vessels (ovals). Magnification × 40. AS, aortic valve stenosis; HF, heart failure; PEF, preserved ejection fraction.
The biomechanical analysis was performed in myocardial samples from 20 patients, in the same mysial and nonmysial regions used for the assessment of CVF. These 20 patients represent the last patients recruited, once the atomic force microscopy procedure was thoroughly tested. Sections (14μm) were attached to positively charged glass slides and were submitted to a process of decellularization for 10minutes in 1% sodium dodecyl sulfate and washed with Dulbecco phosphate-buffered saline. This incubation is sufficient to completely decellularize the myocardial sections. All the micromechanical measurements were made in a NanoWizard III Atomic Force Microscopy (JPK, Berlin, Germany) on top of a Zeiss Axio Observer (Zeiss, Oberkochen, Germany) inverted optical microscope. Measurements were carried out using tipless cantilevers with a nominal spring constant (k) of 0.03N/m (MLCT, Bruker; Mannheim, Germany) and attached 10μm diameter polystyrene beads. The actual spring constant was calibrated by thermal tuning. From the approach between the tip and the sample we obtained a force vs indentation curve and then fitted the Hertz model for spherical tips for an indentation of 500nm.13 Two hundred force-penetration curves were performed per patient sample. We obtained the effective Young's elastic modulus (YEM), the contact point deflection (doff) and the contact point height (zc) from the following equation:
where d is the cantilever deflection, z the holder height, ν the Poisson's ratio (0.5), kc the cantilever spring constant, and R the tip radius (5μm).In each of these patients, we calculated the average of YEM from the 2 nonmysial regions evaluated and we determined the nonmysial YEM:mysial YEM ratio.
Immunohistochemical StudyThe fraction of myocardial volume occupied by collagen type I and type III fibers (CIVF and CIIIVF, respectively) was determined by immunofluorescence and confocal microscopy in tissue samples from 5 patients, specifically in the 2 mysial and 1 nonmysial regions in which YEM had been previously assessed. These 5 patients were consecutively selected, excluding only those cases in which the tissue sample seemed to be inappropriate after macroscopic evaluation.
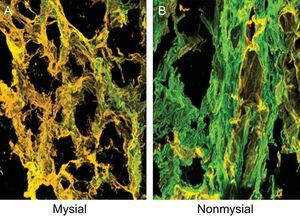
The sections were stained with anticollagen type I and type III monoclonal antibodies (Abcam) (Figure 2) and the amount of fluorescence was quantified. The CIVF:CIIIVF ratio was calculated to assess the relative changes between the 2 types of collagen fibers.
Endomyocardial tissue from 1 patient with severe AS with PEF and symptoms of HF. Sections were stained with specific monoclonal antibodies against collagen types I and III and the fibers were identified in green and yellow, respectively, on confocal microscopy. A: Corresponds to the mysial region of the collagen network. B: Corresponds to the nonmysial region of the collagen network. Magnification × 60. AS, aortic valve stenosis; HF, heart failure; PEF, preserved ejection fraction.
Values are expressed as mean ± standard deviation, median [interquartile range], or number of participants (%). The normal distribution of all continuous variables was tested by use of the Shapiro-Wilk test. Differences between groups were tested by the Student t test for unpaired data. Nonparametric distributed variables were examined after logarithmic transformation. For nonnormally distributed data, a nonparametric test (Mann-Whitney U test) was used. Correlations were estimated by using the Pearson correlation coefficient and univariate regression analyses. The nonmysial:mysial CVF and nonmysial:mysial YEM ratios were multiplied to yield a composite variable reflecting both anatomical and biomechanical properties of the collagen matrix in the nonmysial regions with respect to the mysial regions. The influence of confounding factors on the correlations of interest, considering those that were associated with the E:e’ ratio by univariate regression analysis (systolic blood pressure, fasting glucose, and indexed valve area), as well as LV mass index, age and sex, was excluded by linear multiple regression analyses (with at least 10 participants for each variable in the final models). Additional adjustments were not considered because of the low number of patients. Statistical significance was set at a 2-sided level of 0.05. The analyses were performed using the program STATA (12.1 version).
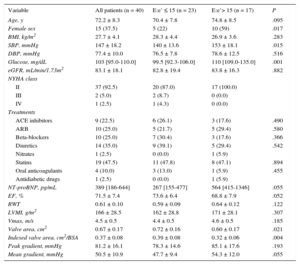
RESULTSTable 1 shows the clinical, biochemical, and echocardiographic characteristics of the whole group of patients, and of patients categorized according to the presence or absence of high LV filling pressures diagnosed on the basis of E:e’ ratio values > or ≤ 15, respectively. As shown in Table 1, patients with E:e’ ratio values > 15 exhibited a higher prevalence of female sex, increased systolic blood pressure, and fasting glucose levels, and a lower crude and indexed valve area than patients with E:e’ ratio values ≤ 15.
Clinical, Biochemical and Cardiac Parameters in Patients With Severe Aortic Valve Stenosis With Preserved Ejection Fraction and Symptoms of Heart Failure, Categorized According to the E:e’ Ratio
| Variable | All patients (n = 40) | E:e’ ≤ 15 (n = 23) | E:e’> 15 (n = 17) | P |
|---|---|---|---|---|
| Age, y | 72.2 ± 8.3 | 70.4 ± 7.8 | 74.8 ± 8.5 | .095 |
| Female sex | 15 (37.5) | 5 (22) | 10 (59) | .017 |
| BMI, kg/m2 | 27.7 ± 4.1 | 28.3 ± 4.4 | 26.9 ± 3.6 | .283 |
| SBP, mmHg | 147 ± 18.2 | 140 ± 13.6 | 153 ± 18.1 | .015 |
| DBP, mmHg | 77.4 ± 10.0 | 76.5 ± 7.8 | 78.6 ± 12.5 | .516 |
| Glucose, mg/dL | 103 [95.0-110.0] | 99.5 [92.3-106.0] | 110 [109.0-135.0] | .001 |
| eGFR, mL/min/1.73m2 | 83.1 ± 18.1 | 82.8 ± 19.4 | 83.8 ± 16.3 | .882 |
| NYHA class | ||||
| II | 37 (92.5) | 20 (87.0) | 17 (100.0) | |
| III | 2 (5.0) | 2 (8.7) | 0 (0.0) | |
| IV | 1 (2.5) | 1 (4.3) | 0 (0.0) | |
| Treatments | ||||
| ACE inhibitors | 9 (22.5) | 6 (26.1) | 3 (17.6) | .490 |
| ARB | 10 (25.0) | 5 (21.7) | 5 (29.4) | .580 |
| Beta-blockers | 10 (25.0) | 7 (30.4) | 3 (17.6) | .366 |
| Diuretics | 14 (35.0) | 9 (39.1) | 5 (29.4) | .542 |
| Nitrates | 1 (2.5) | 0 (0.0) | 1 (5.9) | |
| Statins | 19 (47.5) | 11 (47.8) | 8 (47.1) | .894 |
| Oral anticoagulants | 4 (10.0) | 3 (13.0) | 1 (5.9) | .455 |
| Antidiabetic drugs | 1 (2.5) | 0 (0.0) | 1 (5.9) | |
| NT-proBNP, pg/mL | 389 [186-644] | 267 [155-477] | 564 [415-1346] | .055 |
| EF, % | 71.5 ± 7.4 | 73.6 ± 6.4 | 68.8 ± 7.9 | .052 |
| RWT | 0.61 ± 0.10 | 0.59 ± 0.09 | 0.64 ± 0.12 | .122 |
| LVMI, g/m2 | 166 ± 28.5 | 162 ± 28.8 | 171 ± 28.1 | .307 |
| Vmax, m/s | 4.5 ± 0.5 | 4.4 ± 0.5 | 4.6 ± 0.5 | .185 |
| Valve area, cm2 | 0.67 ± 0.17 | 0.72 ± 0.16 | 0.60 ± 0.17 | .021 |
| Indexed valve area, cm2/BSA | 0.37 ± 0.08 | 0.39 ± 0.08 | 0.32 ± 0.06 | .004 |
| Peak gradient, mmHg | 81.2 ± 16.1 | 78.3 ± 14.6 | 85.1 ± 17.6 | .193 |
| Mean gradient, mmHg | 50.5 ± 10.9 | 47.7 ± 9.4 | 54.3 ± 12.0 | .055 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; BSA, body surface area; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; LVMI, left ventricular mass index; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RWT, relative wall thickness; SBP, systolic blood pressure; Vmax, maximal flow velocity recorded at aortic valve level.
E:e’ ratio indicates the ratio between the maximal early diastolic transmitral flow velocity in diastole and the early mitral annulus velocity in diastole.
Values are expressed as mean ± standard deviation, median [interquartile range], or No. (%).
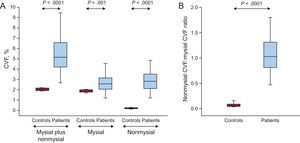
As shown in Figure 3A, increased values of total CVF and of mysial and nonmysial CVF were found in patients compared with control participants, with 3-fold, 2-fold and 38-fold increments, respectively. In accordance with these finding, the nonmysial CVF:mysial CVF ratio was significantly increased in patients compared with controls (Figure 3B). This ratio was slightly higher in patients with E:e’ ratio values > 15 (1.3 ± 0.6) than in patients with E:e’ ratio values ≤ 15 (1.0 ± 0.3), but this difference was not statistically significance (P = .10).
A: Distribution of the volume of myocardial tissue occupied by collagen fibers (CVF) in the mysial region plus the nonmysial region, the mysial region alone, and the nonmysial region alone in control participants (n = 10) and in patients with severe AS with PEF and symptoms of HF (n = 38). B: Distribution of the ratio of the nonmysial CVF to the mysial CVF in controls and patients. Box plots show the 5th and 95th (vertical lines), 25th and 75th (boxes), and 50th (horizontal line) percentile values. AS, aortic valve stenosis; CVF, collagen volume fraction; HF, heart failure; PEF, preserved ejection fraction.
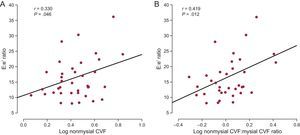
Whereas nonmysial CVF was directly correlated with the E:e’ ratio (Figure 4A) in all patients, mysial CVF was not. The nonmysial CVF:mysial CVF ratio was inversely correlated (r = −0.343; P = .04) with e’ and directly correlated with the E:e’ ratio (Figure 4B) in all patients. Of note, the association between the nonmysial CVF:mysial CVF ratio and the E:e’ ratio was independent of age and sex, and of glucose levels, systolic blood pressure, and the indexed valve area (Table 2). In addition, this association was not influenced by the LV mass index (Table 2).
A: Direct correlation (y = 1.89× + 10.60) between the volume of myocardial tissue occupied by collagen fibers (CVF) in the nonmysial region and the ratio of the maximal early transmitral flow velocity in diastole to the early mitral annulus velocity in diastole (E:e’ ratio) in patients with severe AS with PEF and symptoms of HF (n = 38). B: Direct correlation (y = 6.08× + 9.37) between the ratio of the nonmysial CVF to the mysial CVF and the E:e’ ratio in patients (n = 38). AS, aortic valve stenosis; CVF, collagen volume fraction; HF, heart failure; PEF, preserved ejection fraction.
Adjusted E:e’ Ratio Traits in Relation to the Nonmysial Collagen Volume Fraction:Mysial Collagen Volume Fraction Ratio
| Variable | Covariables | Estimate | 95%CI | P | R2 |
|---|---|---|---|---|---|
| Model 1 | Age and sex | 3.570 | 0.127-7.267 | .048 | 0.333 |
| Model 2 | Glucose, SBP and indexed valve area | 3.804 | 0.117-7.491 | .043 | 0.341 |
| Model 3 | LVMI | 4.683 | 1.042-8.323 | .013 | 0.270 |
95%CI, 95% confidence interval; CVF, collagen volume fraction; LVMI, left ventricular mass index; SBP, systolic blood pressure.
The estimates were calculated in 38 patients.
Estimates are effect sizes associated with a doubling of the nonmysial CVF:mysial CVF. E:e’ ratio indicates the ratio between the maximal early diastolic transmitral flow velocity in diastole and the early mitral annulus velocity in diastole.
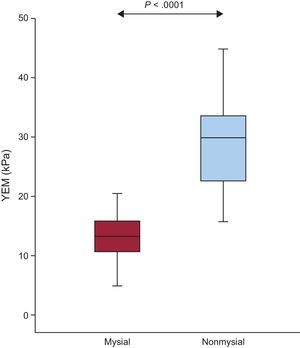
As shown in Figure 5, the value of YEM was significantly higher in the nonmysial region than in the mysial region in patients. In addition, increased values of the nonmysial YEM:mysial YEM ratio were found in patients with E:e’ ratio values > 15 compared with patients with E:e’ ratio values ≤ 15 (2.8 ± 1.6 vs 1.8 ± 0.4; P = .05).
Distribution of the YEM in the mysial and nonmysial regions in patients with severe AS with PEF and symptoms of HF (n = 20). Box plots show the 5th and 95th (vertical lines), 25th and 75th (boxes), and 50th (horizontal line) percentile values. AS, aortic valve stenosis; HF, heart failure; PEF, preserved ejection fraction; YEM, Young's elastic modulus.
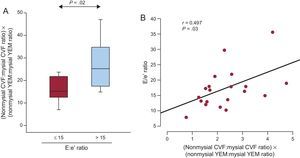
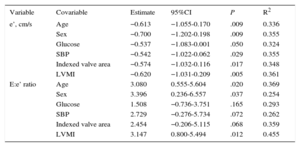
The product nonmysial CVF:mysial CVF ratio by nonmysial YEM:mysial YEM ratio was significantly increased in patients with E:e’ ratio values > 15mmHg compared with patients with E:e’ ratio values ≤ 15 (Figure 6A). The above product was inversely correlated with e’ (r = −0.578; P < .008) and directly correlated with the E:e’ ratio (Figure 6B) in all patients. Interestingly, the associations of the product with e’ were independent of age, sex, glucose, systolic blood pressure, the indexed valve area, and LV mass index (Table 3). In addition, the associations of the product with the E:e’ ratio were independent of age, sex, and LV mass index (Table 3).
A: Distribution of the product nonmysial CVF:mysial CVF ratio by nonmysial YEM:mysial YEM ratio in patients with severe AS with PEF and symptoms of HF (n = 20) categorized according to the ratio of the maximal early transmitral flow velocity in diastole to the early mitral annulus velocity in diastole (E:e’ ratio) cutoff point ≤ (n = 8) or > 15 (n = 12). B: Direct correlation (y = 3.09× + 10.44) between the product nonmysial CVF:mysial CVF ratio by nonmysial YEM:mysial YEM ratio and the E:e’ ratio in patients (n = 20). AS, aortic valve stenosis; CVF, collagen volume fraction; HF, heart failure; PEF, preserved ejection fraction; YEM, Young's elastic modulus.
Adjusted e’ and E:e’ Ratio Traits in Relation to the Product Nonmysial Collagen Volume Fraction:Mysial Collagen Volume Fraction Ratio by Nonmysial Young's Elastic Modulus:Mysial Young's Elastic Modulus Ratio
| Variable | Covariable | Estimate | 95%CI | P | R2 |
|---|---|---|---|---|---|
| e’, cm/s | Age | −0.613 | −1.055-0.170 | .009 | 0.336 |
| Sex | −0.700 | −1.202-0.198 | .009 | 0.355 | |
| Glucose | −0.537 | −1.083-0.001 | .050 | 0.324 | |
| SBP | −0.542 | −1.022-0.062 | .029 | 0.355 | |
| Indexed valve area | −0.574 | −1.032-0.116 | .017 | 0.348 | |
| LVMI | −0.620 | −1.031-0.209 | .005 | 0.361 | |
| E:e’ ratio | Age | 3.080 | 0.555-5.604 | .020 | 0.369 |
| Sex | 3.396 | 0.236-6.557 | .037 | 0.254 | |
| Glucose | 1.508 | −0.736-3.751 | .165 | 0.293 | |
| SBP | 2.729 | −0.276-5.734 | .072 | 0.262 | |
| Indexed valve area | 2.454 | −0.206-5.115 | .068 | 0.359 | |
| LVMI | 3.147 | 0.800-5.494 | .012 | 0.455 |
95%CI, 95% confidence interval; LVMI, left ventricular mass index; SBP, systolic blood pressure.
The estimates were calculated in 20 patients.
e’ indicates early mitral annulus velocity in diastole; E:e’ denotes the ratio between the maximal early diastolic transmitral flow velocity in diastole and the early mitral annulus velocity in diastole.
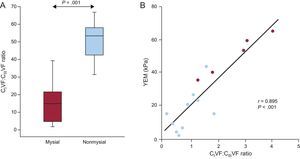
Finally, CIVF was increased by 1.8-fold and CIIIVF was decreased by 3-fold in the nonmysial region compared with the mysial region in patients. As a consequence, the CIVF:CIIIVF ratio was significantly higher in the nonmysial region than in the mysial region (Figure 7A). Of interest, a strong direct correlation was found between the CIVF:CIIIVF ratio and YEM (Figure 7B) in patients.
A: Distribution of the ratio of the volume of myocardial tissue occupied by collagen type I fibers to the volume of myocardial tissue occupied by collagen type III fibers (CIVF:CIIIVF ratio) in the mysial and the nonmysial regions of the myocardium from 5 patients with severe AS with PEF and symptoms of HF. Box plots show the 5th and 95th (vertical lines), 25th and 75th (boxes), and 50th (horizontal line) percentile values. B: Direct correlation (y = 16.93× + 3.50) between the CIVF:CIIIVF ratio and the YEM measured in 2 mysial regions (blue circles) and 1 nonmysial region (red circles) of 5 patients. AS, aortic valve stenosis; CIVF, collagen type I volume fraction; CIIIVF, collagen type III volume fraction; HF, heart failure; PEF, preserved ejection fraction; YEM, Young's elastic modulus.
The main findings of this study are as follows: first, although increased fiber deposition was seen in both the mysial and the nonmysial regions of the myocardial collagen network in patients with severe AS with PEF and symptoms of HF, the increase was much more pronounced in the nonmysial region than in the mysial region; second, nonmysial collagen deposition, but not mysial collagen deposition, was associated with high LV filling pressures in severe AS with PEF and symptoms of HF; third, compared with the mysial region, the nonmysial region in these patients was characterized by increased stiffness and the predominant deposition of collagen type I fibers over collagen type III fibers. These findings suggest that alterations in the anatomical localization, phenotypic composition, and biomechanical properties of collagen tissue may contribute to LV diastolic dysfunction in patients with severe AS and PEF complicated with symptoms of HF.
Components of the Collagen Network and Left Ventricular Diastolic DysfunctionThe collagen network of the myocardium is divided into mysial and nonmysial components that serve various functions.14 In particular, the nonmysial component is the collagen matrix that lies between the endothelium of the myocardium and the epicardium, forming a complex array of collagen fibers. The available evidence indicates that the increase of the nonmysial collagen network is associated with alterations in diastolic function.15,16 In agreement with this possibility, we found that in patients with severe AS with PEF and symptoms of HF an excess of nonmysial collagen was associated with the increase in LV filling pressures, as assessed by the increase in the E:e’ ratio. Of interest, this association was not influenced by LV hypertrophy, indicating the importance of nonmysial collagen accumulation for the compliance properties of the pressure-overloaded left ventricle,17 a notion previously advanced in experimental studies.18–20
A further issue that arises is whether the biomechanical properties of the myocardial collagen network and thereby the myocardial extracellular matrix are influenced by differential expression of fibrillary collagen phenotypes. A study performed in ex-vivo human right atrial tissue demonstrated that, compared with collagen type III fibers, collagen type I fibers had significantly higher YEM.21 Considering the functional implication of this finding, it could be hypothesized that a relative increase in collagen I would therefore confer the myocardial collagen network with a greater stiffness and poor diastolic function. In accordance with this, we found that an association existed between the predominance of collagen type I fibers over collagen type III fibers and the increased stiffness in the nonmysial region of the collagen network in patients with severe AS with PEF and symptoms of HF. This finding might provide the mechanistic link for the previously discussed association between nonmysial collagen deposition and LV diastolic dysfunction.
Clinical ImplicationsWhat are the clinical implications of the reported findings? Long-term follow-up of patients with severe AS after aortic valve replacement suggests that valve replacement may not result in complete reversal of the maladaptive changes that occur within the myocardial extracellular matrix secondary to the pressure overload state. In fact, residual extracellular matrix abnormalities such as myocardial fibrosis are likely responsible for persistent abnormalities in LV diastolic function and increased morbidity and mortality after aortic valve replacement.1 Therefore, there is a need for novel treatments beyond aortic valve replacement in AS patients, namely in those with PEF and symptoms of HF. The development of novel therapies requires the identification of molecular mechanisms that cause the development of diastolic dysfunction in these patients. The current study identifies one mechanism as a potential target: the excessive accumulation of collagen type I in the nonmysial regions of the myocardial collagen network resulting in increased stiffness of the extracellular matrix. In addition, circulating or imaging biomarkers that reflect these changes in myocardial collagen tissue could be used to both improve diagnostic criteria and prognostic assessment in severe AS with PEF and symptoms of HF. In this regard, the possibility exists that, as shown in patients with hypertensive heart disease and HF with PEF,22 changes in serum levels of the tissue inhibitor of matrix metalloproteinases-1 may serve to detect the earliest development of collagen-mediated LV diastolic dysfunction in AS patients, as well as to monitor the efficacy of aortic valve replacement to repair myocardial remodeling in severe AS with PEF and symptoms of HF.
LimitationsThis study has some limitations. First, this was a study involving a relatively small number of patients. In particular, the assessment of collagen phenotypes was performed in a limited number of biopsy samples that were consecutively but not randomly selected. However, because of the nature of the goals under investigation, it was adequately powered. Importantly, no differences were found on comparison of the baseline characteristics among the initial 40 patients, the 20 patients with YEM assessment, and the 5 patients with CIVF and CIIIVF quantification (data not shown). Second, in addition to changes in the ratio between collagen types, collagen quality can also be affected by posttranslational modifications of collagen molecules, particularly increased cross-linking.23 In fact, it has been reported that an excess of collagen cross-linking was associated with increased myocardial stiffness in hypertensive patients with HF and PEF.24,25 Unfortunately, the limited amount of biopsy obtained in this study did not permit evaluation of cross-linked and noncross-linked collagen. Third, myocardial stiffness is also determined by the sarcomeric protein titin, which regulates cardiomyocyte passive tension.26 As recently shown, changes in the phosphorylation state of titin were associated with enhanced titin-dependent myocardial stiffness in hypertensive patients with HFPEF25 and in diabetic patients with aortic stenosis.27 Therefore, although stiffness was measured in decellularized tissue, additional studies in intact tissue are required to assess cardiomyocyte-dependent myocardial stiffness in patients with AS and HFPEF. Fourth, because they are descriptive in nature, the associations found between parameters assessing CVF and YEM and the E:e’ ratio do not establish causality. Fifth, estimation of high filling pressures by evaluation of the E:e’ ratio presents some limitations, in particular in cases of decompensated HF, severe LV dysfunction, large ventricles, conduction abnormalities, or significant mitral regurgitation. However, none of these conditions were present in our study population.
CONCLUSIONSIn conclusion, we report that nonmysial accumulation of collagen fibers, namely of type I, is associated with increased extracellular matrix stiffness and LV filling pressures in severe AS with PEF and symptoms of HF. Albeit descriptive in nature, these findings suggest that an increase in passive stiffness caused by phenotypic and biomechanical changes of the nonmysial collagen network may contribute to the development of diastolic dysfunction and dyspnea in severe AS with PEF.
FUNDINGMinistry of Economy and Competitiveness, Madrid, Spain (Instituto de Salud Carlos III grants RD12/0042/0009 and PI15/01909, and CIBER-CV CB16/11/00483), and the European Commission FP7 Programme, Brussels, Belgium (MEDIA project grant HEALTH-2010-261409 and FIBRO-TARGETS project grant FP7-HEALTH-2013-602904). A.E.A. is supported by a Juan de la Cierva Fellowship (Ministry of Economy and Competitiveness). Ministry of Economy and Competitiveness, “RETOS DE LA SOCIEDAD” grants TEC 2013-48552-C2-2-R. Basque Government (PI 2015-044) Consejería de Educación, Política Lingüística y Cultura.
CONFLICTS OF INTERESTNone declared.
- –
Myocardial fibrosis not only contributes to the progressive impairment of LV diastolic function that may facilitate the development of HF in patients with severe AS and PEF, but may also negatively influence the outcome after aortic valve replacement in these patients.
- –
In this context, it is unknown which characteristics of myocardial fibrosis have an impact on LV diastolic function.
- –
The current study demonstrates, specifically (“or for the first time”), that excessive deposition of collagen type I fibers in the nonmysial region of the myocardial collagen network is primarily responsible for the increase in extracellular matrix stiffness, as assessed by atomic force microscopy, and is associated with LV diastolic dysfunction in severe AS with PEF and symptoms of HF.
- –
The identification of these changes with appropriate biomarkers and their targeting with specific therapies are novel tools to face the medical challenge represented by HF in severe AS with PEF.
The authors thank Sonia Martínez and María J. González for their valuable technical assistance.
S. Ravassa and R. Querejeta jointly supervised this work.