To evaluate the clinical and economic impact of a multidisciplinary program to reduce bleeding events in patients with acute coronary syndrome through optimization of antithrombotic therapy.
MethodsWe designed a preintervention (PRE) and postintervention (POST) quasi-experimental study using a retrospective analysis of 2 cohorts. The first cohort was analyzed to detect correctable measures contributing to bleeding (PRE). Afterward, a quality improvement intervention with a bundle of recommendations was implemented. Finally, a second cohort of patients was evaluated to investigate the impact of the measures on bleeding reduction (POST). The impact on health outcomes was evaluated through comparison of the percentage of in-hospital bleeding events and 30-day readmissions between the 2 cohorts. The economic analysis took into account the costs associated with the implementation of the program and the cost-savings associated with the prevention of bleeding events and 30-day readmissions.
ResultsA total of 677 patients were included (377 in PRE and 300 in POST). The total bleeding rate was reduced after the implementation of the bundled intervention by 29.2% (31.6% in POST vs 22.3% in PRE; OR, 0.62; 95%CI, 0.44-0.88) while 30-day readmission rates were 7.7% in PRE and 5% in POST (P=.20). The estimated avoided cost was €95 113.6 per year, meaning that €10.1 would be obtained in return for each euro invested during the first year and €36.3 during the following years.
ConclusionsThis multidisciplinary program has proven to be effective in reducing bleeding events and is economically attractive.
Keywords
Bleeding is a common complication observed in the treatment of acute coronary syndrome (ACS) and may have a decisive influence on prognosis. Major bleeding is associated with a 3-fold increase in the risk of stroke, a 4-fold increase in the risk of death, and a 5-fold increase in the risk of recurrent myocardial infarction at 30 days.1–3 However, bleeding events are not only associated with prognosis, but also with increases in health expenditures.
In the United States, it has been estimated that bleeding events requiring transfusions in patients with ACS and minor bleeding events associated with percutaneous coronary intervention increased the cost of each event by $12 000 and between $1327 and $7238, respectively.4 A Spanish study showed that the average cost of a bleeding event after ACS was approximately €8000. This cost ranged between €1400 (for a fatal bleeding event) and €9300 (for a bleeding event associated with a reduction of hemoglobin > 3g/dL).5
Factors contributing to increased costs include prolonged hospital stay, additional testing, and admission to intensive care units.5,6
Many factors contribute to the prevention of bleeding, but a key element is the appropriate use of antithrombotic therapy. To achieve this aim, aspects such as treatment individualization according to patient characteristics and the use of drugs with a more favorable profile should be considered.7
The objective of this study was to analyze the clinical and economic impact of a program to reduce bleeding events in patients with ACS through the optimization of antithrombotic therapy.
METHODSThe intervention methods used have been previously described.8 This study was an analytical observational retrospective cohort study with preintervention (PRE phase) and postintervention (POST phase) assessment. We included all adult patients diagnosed with ACS admitted to the cardiology department of a tertiary hospital with 1400 beds and treated with an antithrombotic. Any patient who was readmitted during the same phase of the study was not included again. The drugs used were aspirin, ticlopidine, clopidogrel, prasugrel, abciximab, tirofiban, eptifibatide, unfractionated heparin, enoxaparin, bivalirudin, fondaparinux, and tenecteplase. The patient list was drawn from the hospital record. Exclusion criteria were bypass surgery and patients with no available data source.
To assess the impact of the proposed measures, we compared the PRE phase (January to July 2010) cohort and the POST phase (September 2011 to February 2012) cohort. In the PRE phase, a retrospective analysis was conducted to evaluate the prevalence of in-hospital bleeding events and to identify correctable factors that could have contributed to bleeding events. Clinical history, analytical data (Modulab), and the list of drugs prescribed in the electronic prescription software (Prescriplant) were consulted for each patient. Two pharmacists were in charge of reviewing these documents and registering the data in an Access database. After the data were analyzed the results were presented and discussed with the head of the hospital coronary unit.
The second phase consisted of developing specific actions to reduce bleeding events through the optimization of antithrombotic therapy based on the results of the PRE phase. Cardiologists, nursing staff, and pharmacists designed a bundle of intervention measures with 3 specific actions: a) to reduce the incidence of overdoses of antithrombotics; b) to increase the use of drugs with a more favorable bleeding safety profile (fondaparinux and bivalirudin); and c) to reduce the percentage of combinations of antithrombotics with higher bleeding risk.
These interventions are described in more detail in the results section. All the proposed intervention measures were communicated to other professionals in clinical sessions and visits. This information was also provided in the treatment protocol of the institution.9
Finally, in POST, a second cohort of patients was investigated to assess the prevalence of in-hospital bleeding events by using the same methodology as in the PRE phase.
Sample Size CalculationGiven that the overall rate of bleeding events found in patients with ACS during routine clinical practice has been reported as 30%,10 300 patients would have to be included in each group to decrease this rate by at least 30% with an α=5% and a power (1-β)=80%.
The value of 30% was set taking into account:
- •
The decrease in bleeding events using fondaparinux and bivalirudin in clinical trials.11,12
- •
The extrapolation of these results to the practice of routine care.
- •
The use of other intervention measures.
All statistical analyses were performed using the SPSS software package (version 18) for Windows (SPSS, Chicago, USA) and EPIDAT V 3.1. A P value of < 0.05 was used as a cutoff for statistical significance. Qualitative variables are expressed as a frequency distribution and continuous variables are expressed as mean ± standard deviation. Numerical variables with a nonnormal distribution are expressed as median [interquartile range]. The Kolmogorov-Smirnov test was used to test for normality.
The Student t test for normally distributed variables was used to compare differences between groups. The Levene test for equal variances was used to assess the equality of variances. The nonparametric Mann-Whitney U test was used to compare the variables with a nonnormal distribution. The Pearson chi-squared test or Fisher exact test were used to study the association between categorical variables and the linear association test was used to study qualitative variables with a linear trend per category. To assess the effect of the intervention on reducing bleeding events, relative risk reduction, relative risk, odds ratio (OR), the number needed to treat, and 95% confidence intervals (95%CI) were used.
Multivariate logistic regression analysis was used to study the impact of the intervention while controlling for the following potential confounders: age > 75 years, female sex, body weight < 60kg, diabetes mellitus, chronic anemia, chronic kidney failure, a history of bleeding, percutaneous coronary intervention, and catheterization access site.2,13 The adjusted OR was obtained. The Hosmer-Lemeshow test was used to assess the goodness-of-fit and its calibration and the area under the ROC (Receiver Operating Characteristic) curve was used to assess discriminatory ability.
Cost-benefit AnalysisThe cost-benefit analysis took into account the cost-savings and costs of the implementation of the program.
Cost-savings- •
Prevention of bleeding events: Data from the Spanish study mentioned in the introduction were used. The study included a population from a setting similar to ours.5 In that study, the average cost of a bleeding event in Spain was approximately €1400 for a fatal bleeding event and €9300 for a reduction in hemoglobin > 3g/dL. This information was used to calculate the difference in costs between cohorts by counting bleeding events that met any of the 2 definitions multiplied by the estimated cost.
- •
Avoided cost of not using enoxaparin in patients who were prescribed fondaparinux.
Acquisition of a scale (Hill-Rom) coupled to a LIKO lift (Viking model) to measure the weight of bedridden patients and the introduction of fondaparinux and bivalirudin in the hospital pharmacotherapeutic guide.
Analysis of Health OutcomesThe impact on health outcomes was evaluated through the comparison of bleeding events and 30-day readmission between the 2 cohorts. A bleeding event was defined as any bleeding event meeting any of the criteria of the Bleeding Academic Research Consortium (BARC) classification.14 The hospital administration service provided data on the number of readmissions, which were calculated according to the patient's discharge date. Scheduled admissions were not counted.
The study was approved by the Clinical Research Ethics Committee. The hospital did not consider it necessary to obtain informed consent from the patients because the aim of the study was to improve care based on scientific and organizational evidence.
RESULTSThe study included 692 patients (382 in the PRE phase and 310 in the POST phase). In total, 15 patients had to be excluded because the paper medical history was not available. Thus, the final sample size was 377 patients in the PRE phase and 300 in the POST phase.
Preintervention PhaseIn the PRE phase, most of the patients were men (70.6%) and the mean age was 67 years. The most frequent bleeding event risk factor was percutaneous coronary intervention (79.3%), followed by the use of the femoral artery (51.2%) (Table 1). Correctable factors contributing to bleeding events were identified as:
- •
Overdosing with an antithrombotic: in the PRE phase, 17.8% of patients were overdosed with at least 1 drug (Table 2). For this reason, the institutional protocol was revised to define in detail the standard pattern of antithrombotic use on the basis of the main clinical practice guidelines and technical specifications.15,16 In addition, it was found that the doses of antithrombotics (whose dosage depends on body weight) were frequently calculated by the approximate visual estimation of weight. However, because it has been shown that this method is imprecise,17 in the absence of an appropriate method for body weight determination, the patient or the relatives should be asked directly. Thus, weight was measured by the use of a lift coupled to a scale. In the PRE phase, weight was known in 67.4% of the patients.
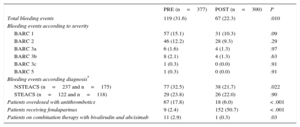
Table 2.Total Bleeding Events and Effectiveness of Interventions in Both Patient Cohorts
PRE (n=377) POST (n=300) P Total bleeding events 119 (31.6) 67 (22.3) .010 Bleeding events according to severity BARC 1 57 (15.1) 31 (10.3) .09 BARC 2 46 (12.2) 28 (9.3) .29 BARC 3a 6 (1.6) 4 (1.3) .97 BARC 3b 8 (2.1) 4 (1.3) .63 BARC 3c 1 (0.3) 0 (0.0) .91 BARC 5 1 (0.3) 0 (0.0) .91 Bleeding events according diagnosis* NSTEACS (n=237 and n=175) 77 (32.5) 38 (21,7) .022 STEACS (n=122 and n=118) 29 (23.8) 26 (22.0) .90 Patients overdosed with antithrombotics 67 (17.8) 18 (6.0) < .001 Patients receiving fondaparinux 9 (2.4) 152 (50.7) < .001 Patients on combination therapy with bivalirudin and abciximab 11 (2.9) 1 (0.3) .03 BARC, Bleeding Academic Research Consortium; NSTEACS, non—ST-segment elevation acute coronary syndrome; POST, postintervention; PRE, preintervention; STEACS, ST–segment elevation acute coronary syndrome.
Data are expressed as no. (%).
- •
The use of drugs with a more unfavorable safety profile regarding bleeding events and alternatives with a lower risk of bleeding events.
- –
Enoxaparin in patients with non—ST-segment elevation acute coronary syndrome and high risk of bleeding according to the CRUSADE scale. In the PRE phase, 97.6% of the patients received enoxaparin treatment. However, the use of fondaparinux in this subgroup of patients has been demonstrated to have a reduced risk of bleeding.11 Only 2.4% of patients were treated with fondaparinux in the PRE phase.
- –
Unfractionated heparin in combination with abciximab. The alternative to this combination (bivalirudin) has shown a decreased risk of bleeding without reduced effectiveness.12 In the PRE phase, 17.0% of the patients received treatment with unfractionated heparin and abciximab and 8.7% with bivalirudin.
- –
- •
The use of combinations of antithrombotics with a higher risk of bleeding, such as the combination of abciximab and bivalirudin. In the PRE phase, 11 patients received treatment with this combination. However, the clinical practice guidelines recommend that its use should be restricted and provisional because the systematic use of bivalirudin and a glycoprotein IIb/IIIa inhibitor has a demonstrated increased risk of bleeding, but without greater efficacy compared with bivalirudin alone.12
Baseline Characteristics of Patients and Risk Factors for Bleeding Events in Both Patient Cohorts
| PRE (n=377) | POST (n=300) | P | |
|---|---|---|---|
| Age, y | 67.2 ± 13.8 | 67.6 ± 13.4 | .67 |
| Sex | .79 | ||
| Men | 266 (70.6) | 208 (69.3) | |
| Women | 111 (29.4) | 92 (30.7) | |
| Body weight, kg | 75.4 ± 13.7 | 77.2 ± 15.0 | .15 |
| STEACS | 122 (32.4) | 118 (39,3) | .07 |
| Hypertension | 240 (63.7) | 208 (69.3) | .14 |
| Hypercholesterolemia | 212 (56.2) | 186 (62.0) | .15 |
| Diabetes mellitus | 110 (29.2) | 80 (26.7) | .53 |
| Smoker | .24 | ||
| No | 141 (37, 4) | 129 (43.0) | |
| Quitter | 105 (27.8) | 77 (25.7) | |
| Yes | 125 (33.2) | 92 (30.7) | |
| Unknown | 6 (1.6) | 2 (0.7) | |
| Chronic kidney failure | 33 (8.8) | 35 (11.7) | .26 |
| History of bleeding | 20 (5.3) | 9 (3.0) | .20 |
| Chronic anemia | 19 (5.0) | 12 (4.0) | .65 |
| Percutaneous coronary intervention | 299 (79.3) | 240 (80.0) | .90 |
| Femoral vein | 193 (51.2) | 131 (43.7) | .25 |
| Place of admission | < .001 | ||
| Coronary unit | 294 (78.0) | 189 (63.0) | |
| Nonintensive cardiology unit | 75 (19.9) | 111 (37.0) | |
| Other | 8 (2.1) | 0 (0.0) |
| Drug therapy prescribed since the beginning of the ischemic episode | |||
|---|---|---|---|
| Antiplatelet agents | 377 (100) | 300 (100) | – |
| Aspirin | 364 (96.6) | 294 (98.0) | .37 |
| Ticlopidine | 1 (0.3) | 0 (0.0) | .91 |
| Clopidogrel | 358 (95.0) | 297 (99.0) | .006 |
| Prasugrel | 0 (0.0) | 10 (3.3) | .001 |
| GPIIb/IIIa inhibitors | |||
| Abciximab | 86 (22.8) | 73 (24.3) | .71 |
| Tirofiban | 12 (3.2) | 2 (0.7) | .04 |
| Eptifibatide | 1 (0.3) | 1 (0.3) | .58 |
| Antiaggregants | 345 (91.5) | 286 (95.3) | .07 |
| Unfractionated heparin | 264 (70, 0) | 235 (78.3) | .019 |
| Enoxaparin | 231 (61.3) | 130 (43.3) | < .001 |
| Fondaparinux | 6 (1.6) | 85 (28,3) | < .001 |
| Bivalirudin | 33 (8.8) | 10 (3.3) | .007 |
| Fibrinolytics: tenecteplase* | 35 (28,2) | 22 (18.5) | .10 |
GPIIb/IIIa, glycoprotein IIb/IIIa; POST, postintervention; PRE, preintervention; STEACS, ST-segment elevation acute coronary syndrome.
Data are expressed as no. (%) or mean ± standard deviation.
The baseline characteristics of the patients and the presence of bleeding risk factors in POST were similar to those in the PRE phase (Table 1). Regarding the degree of acceptance of the proposed intervention measures, it was found that:
- •
The percentage of overdosed patients decreased by 66.3% in POST (17.8% vs 6.0%; P < .001). The percentage of patients with known weight increased from 67.4% to 88.7% (P<.001) (Table 2).
- •
Regarding the use of drugs with a more favorable safety profile:
- –
The percentage of patients treated with fondaparinux increased in POST (2.4% vs 50.7%; P<.001), while the use of enoxaparin decreased (97.6% vs 49.3%; P<.001) (Table 2).
- –
Only 3.3% of patients were treated with bivalirudin (P=.007), while the combination of unfractionated heparin and unfractionated abciximab was 21.3% (P=.019) in POST.
- –
- •
Regarding the use of combinations of drugs with an increased risk of bleeding, only 1 patient (0.3%) was treated with the combination of bivalirudin and abciximab (P=.03) (Table 2) in POST.
The overall percentage of in-hospital bleeding events in POST was reduced by 29.2% (31.6% PRE vs 22.3% POST; OR, 0.62; 95%CI, 0.44-0.88) (Table 2). The adjusted OR was 0.58 (95%CI, 0.40-0.85). The result of the Hosmer-Lemeshow test was not statistically significant (P=.36) and the area under the curve was 0.66.
Regarding the number needed to treat, 11 patients needed intervention measures to prevent a bleeding event (95%CI, 7-39). The readmission rate was 7.7% in the PRE phase and 5% in the POST phase (P=.20) (14 readmissions in the PRE phase and 8 readmissions in the POST phase were for cardiovascular reasons).
Cost-benefit AnalysisCost-savings- •
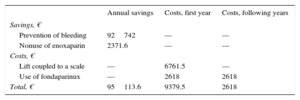
Prevention of bleeding events. In the PRE phase (January to July 2011), there was 1 fatal bleeding event (€1400) and 15 bleeding events associated with a reduction in hemoglobin > 3g/dL (15 × €9300=€139 500). Total cost/mo: €20 128.57. In the POST phase (September 2011 to February 2012), there were no fatal bleeding events, but there were 8 bleeding events associated with a reduction in hemoglobin > 3g/dL (8 × €9300=€74 400). Total cost/mo: €12 400.
Therefore, the cost difference between 1 period and another was €92 742 per year (Table 3). All BARC 3 bleedings were recorded as a bleeding event associated with a reduction in hemoglobin > 3g/dL.
- •
Avoided costs due to not using enoxaparin. The use of fondaparinux decreased the use of enoxaparin. If the cost of enoxaparin 60mg (unit cost in laboratory selling price + VAT: €4.9) is taken into account, and given that the average weight of patients in the POST phase with fondaparinux was 73.7 kg, 242 doses of enoxaparin were avoided, thus saving €2371.6 per year (Table 3). This figure was calculated on the basis that about half of the patients were older than 75 years (0.75mg/kg/dose) and 6 patients had a glomerular filtration rate < 30mL/min (1mg/kg/day).
Economic investments included the purchase of a lift (total price + VAT=€6761.5) and the inclusion of fondaparinux in the hospital pharmacotherapeutic guide (unit cost in laboratory selling price + VAT: €8.5). In the POST phase, 154 doses of fondaparinux were used at a cost of €2618 per year (Table 3).
The cost of bivalirudin was not taken into account because it was prescribed less in the POST phase.
Therefore, the estimated avoided cost was €95 113.6 per year. The annual return on investment shows that savings of €10.1 would be obtained for each euro invested in the intervention program in the first year and €36.3 in the following years (Table 3).
DISCUSSIONThe multidisciplinary program to optimize antithrombotic therapy has proven to be effective in reducing bleeding events and is cost-effective.
Interventions were effective in reducing overdoses with antithrombotics by the introduction of fondaparinux in the therapeutic arsenal used to treat non—ST-segment elevation ACS and by limiting the combined use of bivalirudin and abciximab. These factors were associated with a lower risk of bleeding events. After the implementation of the program, there was a significant reduction in bleeding events and in the numerical rate of readmissions.
The only intervention that was not implemented was the inclusion of bivalirudin as an alternative to the combination of unfractionated heparin and abciximab, possibly due to the high cost of the drug and uncertainty among clinicians concerning its benefits. This attitude may have been the result of the limitations found in the Acute Catheterization and Urgent Intervention Triage Strategy trial in patients with non—ST-segment elevation ACS.18
The risk of bleeding events found in the present study may seem high (31.8%). However, most of the studies that have assessed bleeding events in ACS patients have been clinical trials, which generally tend to underestimate the true severity of the problem. Since the publication of the BARC bleeding classification in 2011, an increasing number of studies have used this methodology to define bleeding events. The overall percentage of bleeding events in our study (31.8%) was similar to that of a study published in 2013 (30%), which was conducted in clinical practice. However, it should be noted that the authors not only assessed in-hospital bleeding events, but bleeding at 6 months.10 More recent observational studies, such as that of Alexopoulos et al.19 have found an even higher percentage of total bleeding events (about 49%), which may have been due to the inclusion of only patients undergoing percutaneous coronary intervention and treated with prasugrel and ticagrelor.
The reduction in bleeding events according to the type of ACS was greater in non—ST-segment elevation ACS. This result could be explained in 2 ways: a) the use of fondaparinux in patients with non—ST-segment elevation ACS in the POST phase; and b) the increased use of combinations of antithrombotics in patients with ST-segment elevation ACS, regardless of the study period (around 30% received treatment with 5 or more drugs vs 15% of those diagnosed with non—ST-segment elevation ACS).
Due to their simplicity of implementation and low cost, we consider the proposed measures to be accessible to most services:
- •
Rethinking the protocols did not involve a direct economic investment.
- •
The introduction of fondaparinux for the treatment of patients at high risk of bleeding events did not involve increased costs.
- •
Most hospital units have scales available for the measurement of body weight. However, educational guidelines to raise awareness of the importance of not visually estimating body weight are often lacking.
The novelty of this study lies in its attempt to rethink standard antithrombotic therapy from the point of view of the most basic aspects. That is, to assess the treatment protocol point by point to detect care practices that are fully integrated within the work routine, but that could increase the risk of bleeding events in a hidden manner.
Although many studies have emphasized the importance of bleeding risk stratification in decisions on the therapeutic treatment of patients with ACS,20,21 we have found no studies that have investigated the impact of a bundle of measures to reduce bleeding events in patients with ACS. The concept of a care bundle is to apply a set of evidence-based measures (usually between 3 and 5) with a synergistic effect when applied together. An example of this is the known Surviving Sepsis Campaign to reduce mortality in patients with sepsis.22
LimitationsThe present study is limited by its retrospective design. Its most important bias lies in the difficulty of validating the information obtained from medical records. However, the reliability of the study is increased by the comparison of 2 patient groups given that the magnitude of bias is similar in both groups. Another aspect associated with the limitations of retrospective studies was the inability to record ischemic and bleeding risk a posteriori, which would undoubtedly have been very useful information.23 However, it should be clarified that the assessment of GRACE and CRUSADE scores is considered in the Cardiology Service Protocols of our hospital for the indication for antiplatelet therapy in patients with ACS.9 Secondly, savings were estimated using a partial extrapolation of the data published by Soldevila et al.5 rather than by using data from our own hospital. The estimation was done in this way because the aim of the study was to obtain an approximate cost-benefit analysis rather than an exhaustive pharmacoeconomic evaluation. Furthermore, we consider it appropriate to use data from the aforementioned study because it was performed under similar conditions (reperfusion strategies used, antithrombotic therapy used, national study). Third, the frequent current use of prasugrel and ticagrelor may suggest that the bleeding event rate could have been higher than that reported in the study. However, this eventuality would not diminish the effectiveness of the intervention in reducing bleeding events. Finally, it was difficult to demonstrate a statistical difference in less prevalent events, such as readmissions, because a very large sample size would have been needed in each group.
CONCLUSIONSThe present multidisciplinary program has proven to be effective in reducing bleeding events in patients with ACS and is economically attractive.
FUNDINGCNIC is funded by the Ministry of Economy and Competitiveness (MINECO) and the ProCNIC Foundation and is a Severo Ochoa Center of Excellence (MINECO SEV-2015-0505).
CONFLICTS OF INTERESTH. Bueno receives funding from Instituto de Salud Carlos III (PIE16 / 00021) and from Astra-Zeneca, BMS, Janssen, and Novartis for research projects and has received payments for consultancy and conferences, or assistance to attend conferences held by Abbott, Astra- Zeneca, Bayer, BMS-Pfizer, Ferrer, MedScape-the heart.org, Novartis, and Servier.
- –
Bleeding is a common complication in ACS patients.
- –
Bleeding is not only associated with prognosis, but also involves an increase in health costs.
- –
The appropriate use of antithrombotic therapy is a key element in its prevention.
- –
There is still room to improve the use of antithrombotics in patients with ACS.
- –
Optimization of ACS treatment through a multidisciplinary improvement intervention significantly reduced the bleeding event rate and the numerical rate of readmissions at 30 days.
- –
The estimated avoided cost of this improvement intervention is economically attractive.
- –
Due to their simplicity of implementation and low cost, the proposed measures are accessible to most services.
This project was made possible due to a grant awarded by the Ministry of Health, Social Policy, and Equality (Order SAS/2377/2010) and was a collaborative effort on the part of the coronary unit, the cardiac catheterization laboratory, the pharmacy service, the archive service, and Alfonso Cuadrado.