Hereditary transthyretin amyloidosis (ATTRv) is a systemic disease with an autosomal dominant inheritance pattern, and more than 100 pathogenic variants have been described. The prevalence of ATTRv varies widely among regions, and the disease is endemic in certain geographical areas due to founder effects. For instance, the p.Val50Met variant is endemic in Povoa de Varzim (Portugal), Västerbotten (Sweden), and Mallorca (Spain).1 The p.Glu109Lys variant is the third most frequent mutation in Spain and is defined by early age of onset, mixed cardiac and neurologic involvement, and an overall poor prognosis. A recent study detected a founder effect of the variant believed to have originated in south-east Spain.2,3
ATTRv is an optimal disease for undertaking screening efforts due to its significant impact on health, the long latent period of the disease, and the availability of noninvasive screening tests and effective disease-modifying therapies.1,4 Based on these characteristics, we sought to examine the feasibility of implementing a screening program in the town of origin of the p.Glu109Lys variant.
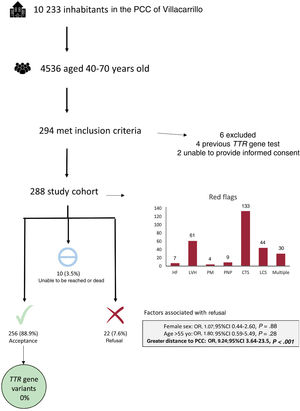
For this purpose, a prospective study was designed to offer genetic screening for the transthyretin (TTR) gene to inhabitants of Villacarrillo at risk of ATTRv. Inclusion criteria were age 40 to 70 years and the presence of at least 1 of the following clinical red flags for ATTRv: a diagnosis of heart failure not explained by ischemic cardiomyopathy or valvular heart disease; left ventricular hypertrophy (≥12mm) on echocardiography; pacemaker implantation due to conduction disturbances; signs/symptoms of peripheral neuropathy defined by the presence of paresthesia, sensory loss or neuropathic pain in the absence of other neurological disease; carpal tunnel syndrome; and lumbar spinal stenosis. Patients with a previous TTR genetic study or with any conditions that disqualified them from taking an informed decision on genetic testing were excluded. Identification of candidates was manually performed by remote review of the electronic medical records of Villacarrillo primary care center (PCC). Patients fulfilling the inclusion criteria were contacted by the PCC staff by telephone or during routine visits to invite them to participate. Patients willing to participate signed an informed consent form, received genetic counseling, and provided a saliva sample for genetic testing of the TTR gene. Genetic results were provided to participants by the PCC medical staff.
All inhabitants registered at Villacarrillo PCC in February 2022 were included (10 233 persons). The clinical records of the 4536 individuals aged 40-70 years old were analyzed. After a medical records review, 294 inhabitants were identified as possible candidates but 6 were excluded due to previous TTR gene testing (n=4) or were disqualified due to their inability to make an informed decision (n=2). The final study cohort included 288 patients (6.4% of screened participants). The mean age was 59.1±7.5 years and 164 (56.9%) were female. Among clinical red flags, carpal tunnel syndrome was the most frequent (n=133, 46.2%), followed by left ventricular hypertrophy (n=61, 21.2%) and lumbar spinal stenosis (n=44, 15.3%). Thirty patients (10.4%) had ≥ 2 red flags. A total of 256 individuals (88.9%) underwent genetic testing, while 22 participants (7.6%) refused to participate and 10 (3.5%) were not included because they could not be reached (n=6, 2.1%) or because they died before contact (n=4, 1.4%). An exploratory analysis found that patients not living in the main town of the municipality had an increased probability of rejecting screening (OR, 9.24; 95%CI, 3.64-23.5, P<.001). Genetic testing was successfully performed in all patients with no incidents but none of the patient were identified as carrying the p.Glu109Lys variant or any other pathogenic variant (figure 1).
To the best of our knowledge, this is the first study to perform a population screening program targeting a hereditary cardiac disease within a specific region in Spain. Our findings demonstrate that this approach is feasible and has a high acceptance rate among potential participants. We propose that 2 factors contributed to the high rate of acceptance. First, the involvement of local medical staff in patient outreach, which may have engendered trust. Second, the use of saliva kits instead of blood collection methods, which facilitated participation. In addition, our results also suggest that proximity plays an important role in acceptance. Our work also shows that ATTR red flags are very common in the general population, as they were present in 6% of participants aged 40 to 70 years. This finding suggests that future projects designed to screen for ATTR in the general population may benefit from a more targeted approach focusing on more specific red flags or a combination of common red flags to increase specificity and cost-effectiveness.
Population genetic screening programs are expected to grow exponentially in the coming years due to cheaper access to genetic studies. Overall, pilot programs are focusing on newborns, and although they include a myriad of diseases, inherited cardiac diseases have not usually been included.5 Population genetic screening for ATTR has been proposed, particularly in countries with a high prevalence of the black population, as the pathogenic p.Val142Ile variant affects 3% to 4% of black individuals in the US.6 These programs might be based on automatic big data retrieval from electronic health records designed to detect specific red flags.
In summary, despite the negative result of our screening project, our experience provides valuable insights about the feasibility of genetic screening programs and the possible barriers to their implementation in real life scenarios.
FUNDINGThis work was supported by an investigator-initiated research grant from Alnylam Pharmaceuticals, Inc.
ETHICAL CONSIDERATIONSThe study was approved by the ethics committee of Hospital Universitario Puerta de Hierro and Hospital Universitario de Jaén and participants provided informed consent. Sex and gender were not considered according to SAGER.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tool was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSF. de Frutos and I. Caraballo Ramos are co-first authors. F. de Frutos and P. García-Pavía designed the study. F. de Frutos, I. Caraballo Ramos, V. Martínez Chaves, A.M. Corral Azor, and M.S. Berchíd Débdi collected the data. F. de Frutos and P. García-Pavía drafted the manuscript. I. Caraballo Ramos, V. Martínez Chaves, A.M. Corral Azor, and M.S. Berchíd Débdi critically reviewed the manuscript. P. García-Pavía obtained funding.
CONFLICTS OF INTERESTP. García-Pavía is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. The rest of authors have not conflicts of interest.