Late gadolinium-enhanced cardiac magnetic resonance (LGE-CMR) allows noninvasive detection of left atrial fibrosis in patients with atrial fibrillation (AF). However, whether the same methodology can be used in the right atrium (RA) remains unknown. Our aim was to define a standardized threshold to characterize RA fibrosis in LGE-CMR.
MethodsA 3 Tesla LGE-CMR was performed in 53 individuals; the RA was segmented, and the image intensity ratio (IIR) calculated for the RA wall using 1 557 767 IIR pixels (40 994±10 693 per patient). The upper limit of normality of the IIR (mean IIR+2 standard deviations) was estimated in healthy volunteers (n=9), and patients who had undergone previous typical atrial flutter ablation (n=9) were used to establish the dense scar threshold. Paroxysmal and persistent AF patients (n=10 each) were used for validation. IIR values were correlated with a high-density bipolar voltage map in 15 patients undergoing AF ablation.
ResultsThe upper normality limit (total fibrosis threshold) in healthy volunteers was set at an IIR = 1.21. In the postablation group, 60% of the maximum IIR pixel (dense fibrosis threshold) was calculated as IIR = 1.29. Endocardial bipolar voltage showed a weak but significant correlation with IIR. The overall accuracy between the electroanatomical map and LGE-CMR to characterize fibrosis was 56%.
ConclusionsAn IIR > 1.21 was determined to be the threshold for the detection of right atrial fibrosis, while an IIR > 1.29 differentiates interstitial fibrosis from dense scar. Despite differences between the left and right atria, fibrosis could be assessed with LGE-CMR using similar thresholds in both chambers.
Keywords
Atrial fibrosis is a determinant in the pathogenesis of atrial fibrillation (AF). Technical advances in recent years have enabled noninvasive characterization of atrial fibrosis by means of late gadolinium-enhanced cardiac magnetic resonance (LGE-CMR),1 fostering numerous potential clinical applications. LGE-CMR may be useful to personalize AF ablation with both first2 and re-do3 procedures benefitting from previous characterization of atrial fibrosis.4 Discontinuities (gaps) in anatomical lesions induced by ablation have been shown to predict AF recurrences.5 Finally, LGE-CMR could aid in selecting patients needing chronic anticoagulation for primary prevention of stroke.6 However, the lack of standardized algorithms for fibrosis assessment and their variable reproducibility have limited the uptake and widespread use of LGE-CMR in clinical practice7.
There are anatomical, functional, and molecular differences between the left atrium (LA) and the right atrium (RA). The contribution of the RA to AF pathology remains disputed, but clinical insights underpin a central role of the RA in some patients. The RA may be particularly sensitive to damage inflicted by sleep apnea and other respiratory diseases,8,9 and ectopic foci sustaining AF have occasionally been localized in the RA.10 Unfortunately, our knowledge on the contribution of the RA to AF substrate is, at least in part, jeopardized by technical limitations. For example, efforts in recent years have been directed towards the noninvasive identification of atrial fibrosis in the LA, but no study has yet tested whether similar algorithms are applicable to the RA. Few small studies have employed CMR to measure fibrosis in the RA, including a case series.11 In patients with AF and sinus node dysfunction, Akoum et al.12 found that fibrosis burden was higher in the left than in the right atrium. However, these algorithms had never been validated for RA fibrosis assessment.
Our objective was to define and validate a standardized, systematic, reproducible, and robust method to identify myocardial fibrosis in the RA by mean of LGE-CMR.
METHODSThe study protocol conforms to the Declaration of Helsinki and was approved by the research ethics committee of our institution (HCB/2018/0382). All patients provided signed informed consent. The data that support the findings of this study are available from the corresponding author, upon reasonable request. A comprehensive description of the methods is provided in the supplementary data.
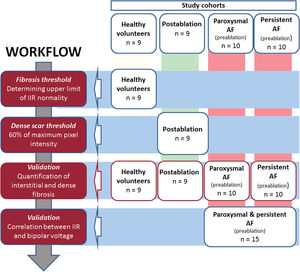
Study design and cohorts under studyA systematic, sequential workflow13 was used in different cohorts (figure 1) to determine RA wall intensity, differentiating healthy from fibrotic tissue as well as interstitial fibrosis from dense scar. This descriptive technical study was meant to lay the foundations for the interpretation of fibrosis measured by LGE-CMR in RA (figure 2).
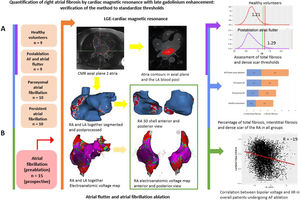
Central illustration. Quantification of right atrial fibrosis by cardiac magnetic resonance with late gadolinium enhancement. A: assessment of RA total fibrosis and dense scar thresholds in healthy volunteers and patients with typical atrial flutter and AF ablation in the same procedure, respectively. Application of the obtained thresholds to paroxysmal and persistent AF groups. B: correlation between LGE-CMR and electroanatomical map in a prospective group of patients undergoing AF ablation. AF, atrial fibrillation; CMR, cardiac magnetic resonance; IIR, image intensity ratio; LA, left atrium; LGE, late gadolinium enhancement; RA, right atrium.
A total of 53 individuals who had undergone LGE-CMR were included in consecutive stages of this study. Initially, thresholds to identify fibrosis in the RA were determined in healthy volunteers (individuals aged 18-30 years who had been recruited to assess LA fibrosis threshold,13 n = 9) and patients who had undergone typical atrial flutter and AF ablation in the same procedure (n = 9, LGE-CMR performed 3 months postablation). Later, patients with paroxysmal (n = 10) or persistent (n = 10) AF were used for validation (LGE-CMR obtained 2 weeks before the ablation procedure). Finally, correlation between the image intensity ratio (IIR) and the electroanatomical bipolar voltage map (EAM) was evaluated in an additional prospective cohort of patients undergoing a first AF ablation procedure (n = 15).
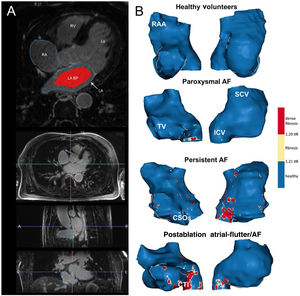
Both the RA and LA were segmented from 3 Tesla (T) LGE-CMR (Magnetom Prisma Siemens Healthcare, Germany) images with ADAS 3D software (figure 3), and 3D shells were built. Signal intensity from each pixel of the RA wall was normalized to the mean LA blood intensity to calculate the IIR. All IIR values were represented in histograms, and the total fibrosis threshold was set as the mean IIR value in the healthy volunteer group plus 2 standard deviations (SD), which ensured that ≈ 97.5% of all pixels in the healthy volunteers group fell below this threshold. The dense scar threshold was defined as the IIR value corresponding to 60% of the maximum normalized intensity pixel in the RA of patients who had undergone cavotricuspid isthmus ablation, as previously defined in the LA.3 Overall, healthy tissue, interstitial fibrosis, and dense scar were derived from IIR values.
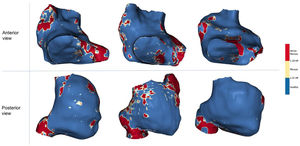
A: postprocessing of LGE-CMR images. In the upper panel, the RA and LA contours were drawn manually in an axial plane; the LA blood pool is shown in red. The 3 lower panels show axial, sagittal and coronal planes of a CMR during RA segmentation. B: anteroseptal (right) and posterolateral (left) views of representative examples of the RA in all the study groups. AF, atrial fibrillation; BP, blood pool; CSOs, coronary sinus ostium; CTI, cavo-tricuspid isthmus; ICV, inferior cava vein; LA, left atrium; LV, left ventricle; RA, right atrium; RAA, right atrium appendage; RV, right ventricle; SCV, superior cava vein; TV, tricuspid valve.
Finally, in 15 consecutive patients undergoing AF ablation, bipolar voltage in an intraprocedural high-density point-by-point EAM of the RA (Lasso or Pentarray catheters, CARTO 3, Biosense-Webster, USA) was correlated to IIR values. Only EAM points projected on the CMR shell less than 10mm apart were used. Standard voltage thresholds of 0.1mV and 0.5mV were used to characterize atrial dense scar, interstitial fibrosis, and healthy tissue.
Statistical analysisContinuous variables are shown as mean±SD or median [interquartile range]) unless otherwise stated, and groups were compared with 1-way ANOVA. The correlation between IIR and EAM was assessed using the Pearson correlation coefficient (r), and a generalized linear mixed model with random intercept accounted for repeated IIR measurements per patient. A 2-sided type I error of 5% was used for all tests. All analyses were performed using R v3.5.1 (R project for Statistical Computing).
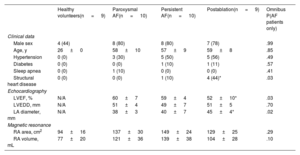
RESULTSBaseline characteristics of the populationThe characteristics of the 4 study groups are shown in table 1. Most participants were male (71%). Young individuals with no risk factors were recruited for the healthy volunteer group. AF groups included middle-aged individuals with a similar burden of cardiovascular risk factors; structural heart disease was uncommon. Echocardiography was only available for AF patients and showed mild LA anteroposterior diameter enlargement (41 ± 6mm). CMR showed a progressive enlargement of the RA from healthy volunteers to persistent AF.
Characteristics of all the cohorts
| Healthy volunteers(n = 9) | Paroxysmal AF(n = 10) | Persistent AF(n = 10) | Postablation(n = 9) | Omnibus P(AF patients only) | |
|---|---|---|---|---|---|
| Clinical data | |||||
| Male sex | 4 (44) | 8 (80) | 8 (80) | 7 (78) | .99 |
| Age, y | 26 ± 0 | 58 ± 10 | 57 ± 9 | 59 ± 8 | .85 |
| Hypertension | 0 (0) | 3 (30) | 5 (50) | 5 (56) | .49 |
| Diabetes | 0 (0) | 0 (0) | 1 (10) | 1 (11) | .57 |
| Sleep apnea | 0 (0) | 1 (10) | 0 (0) | 0 (0) | .41 |
| Structural heart disease | 0 (0) | 0 (0) | 1 (10) | 4 (44)* | .03 |
| Echocardiography | |||||
| LVEF, % | N/A | 60 ± 7 | 59 ± 4 | 52 ± 10* | .03 |
| LVEDD, mm | N/A | 51 ± 4 | 49 ± 7 | 51 ± 5 | .70 |
| LA diameter, mm | N/A | 38 ± 3 | 40 ± 7 | 45 ± 4* | .02 |
| Magnetic resonance | |||||
| RA area, cm2 | 94 ± 16 | 137 ± 30 | 149 ± 24 | 129 ± 25 | .29 |
| RA volume, mL | 77 ± 20 | 121 ± 36 | 139 ± 38 | 104 ± 28 | .10 |
AF, atrial fibrillation; LA, left atrium; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction.
Unless otherwise specified, values are expressed as count and percentage (qualitative variables) or as mean±standard deviation (quantitative variables).
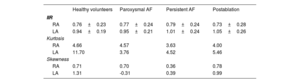
Overall, standardized LGE-CMR intensity values (ie, IIR) of 2 283 069 pixels were obtained from both atria of all participants: 1 557 767 pixels from the RA (40 994 ± 10 693 pixels per patient), and 725 302 pixels from the LA (19 087 ± 11 414 pixels per patient). When all participants were analyzed together, the average IIR was higher in the LA than in the RA (IIR 0.99; 95% confidence interval [95%CI], 0.97-1.02; vs 0.77; 95%CI, 0.74-0.79; respectively; P<.0001) (table 2).
Histogram descriptive values for the image intensity ratio of right and left atria
| Healthy volunteers | Paroxysmal AF | Persistent AF | Postablation | |
|---|---|---|---|---|
| IIR | ||||
| RA | 0.76±0.23 | 0.77±0.24 | 0.79±0.24 | 0.73±0.28 |
| LA | 0.94±0.19 | 0.95±0.21 | 1.01±0.24 | 1.05±0.26 |
| Kurtosis | ||||
| RA | 4.66 | 4.57 | 3.63 | 4.00 |
| LA | 11.70 | 3.76 | 4.52 | 5.46 |
| Skewness | ||||
| RA | 0.71 | 0.70 | 0.36 | 0.78 |
| LA | 1.31 | -0.31 | 0.39 | 0.99 |
AF, atrial fibrillation; IIR, image intensity ratio; LA, left atrium; RA, right atrium; SD: standard deviation.
IIR values are expressed as mean±standard deviation. Kurtosis and skewness are unitless parameters.
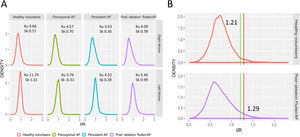
Figure 4A shows the IIR histograms for the RA; LA histograms are provided for reference. We first characterized these histograms to determine how IIR values (ie, atrial fibrosis) were distributed on the RA. In all groups, the IIR histogram was asymmetric, with a long right tail distribution (mean skewness 0.64; a value of 0 denotes a symmetrical distribution) showing that some RA areas had very dense fibrotic patches. Patients with persistent AF had the smallest kurtosis (3.63; a kurtosis of 3 characterizes a normal distribution), revealing a larger dispersion of IIR values. Conversely, the larger kurtosis (4.66), reflecting a lower dispersion of IIR values around the mean, was observed in healthy volunteers.
A: density distribution of IIR values for the RA and LA in all subgroups. B: identification of thresholds for healthy myocardial tissue (green line, measured in a healthy population) and dense scar (red line, measured in previously ablated patients) over RA histograms of all subgroups. AF, atrial fibrillation; IIR, image intensity ratio; Ku, kurtosis; Sk, skewness.
Normal IIR values of the RA myocardium were defined from healthy, young individuals. The upper limit of normality (mean IIR + 2SD) was calculated to be IIR = 1.21 (figure 4B). Therefore, all values with an IIR > 1.21 were considered to represent atrial fibrosis in the RA. Subsequently, the dense scar threshold was established in the group of patients who had undergone cavotricuspid isthmus ablation. As expected, in most cases, the maximum intensity pixel value in the RA was located in the cavotricuspid isthmus (figure 3B, bottom panel), while other high intensity pixels located superior and inferior cava vein, appendage, septum and peri-sinus coronary ostium (figure 5). Sixty percent of the maximum intensity pixel was calculated as IIR = 1.29, which was therefore used to discriminate interstitial fibrosis from dense scar (figure 4B).
Interindividual reproducibility was assessed with the interobserver Lin concordance correlation coefficient in a subset of 10 randomly selected right atria, segmented by 2 different independent observers. Correlation was 0.92 (0.68-0.98) for total fibrosis and 0.97 (0.89-0.99) for dense scar (table 3).
Fibrosis assessment reproducibility
| Total | Paroxysmal AF | Persistent AF | |
|---|---|---|---|
| Lin concordance coefficient (95%CI) | |||
| Total fibrosis | 0.92 (0.68-0.98) | 0.97 (0.63-1.00) | 0.89 (0.20-0.99) |
| Dense scar | 0.97 (0.89-0.99) | 0.91 (0.64-0.98) | 0.98 (0.83-1.00) |
95%CI, 95% confidence interval; AF, atrial fibrillation.
The Lin concordance correlation coefficient was used to assess the agreement for total fibrosis and dense scar (for total sample and by type of AF) in 10 right atria segmented by 2 different independent observers.
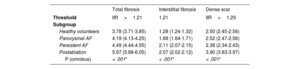
The percentages of total, interstitial fibrosis and dense RA scar pixels were quantified in all groups to validate thresholds. The results are shown in table 4. Healthy volunteers had the lowest total RA fibrosis burden, followed by paroxysmal and persistent AF patients, and the largest amount of RA fibrosis was found in postablation patients. Subsequently, interstitial fibrosis and dense scar were quantified separately. Healthy volunteers and paroxysmal AF patients showed the least interstitial fibrosis, while persistent AF patients showed the most. Finally, postablation showed the largest dense scar, as expected. We found a strong association between interstitial fibrosis burden and the RA area (r = 0.84).
Total fibrosis, interstitial fibrosis and dense scar of the right atrium in all groups
| Total fibrosis | Interstitial fibrosis | Dense scar | |
|---|---|---|---|
| Threshold | IIR>1.21 | 1.21 | IIR>1.29 |
| Subgroup | |||
| Healthy volunteers | 3.78 (3.71-3.85) | 1.28 (1.24-1.32) | 2.50 (2.45-2.56) |
| Paroxysmal AF | 4.19 (4.13-4.25) | 1.68 (1.64-1.71) | 2.52 (2.47-2.56) |
| Persistent AF | 4.49 (4.44-4.55) | 2.11 (2.07-2.15) | 2.38 (2.34-2.43) |
| Postablation | 5.97 (5.88-6.05) | 2.07 (2.02-2.12) | 3.90 (3.83-3.97) |
| P (omnibus) | < .001* | < .001* | < .001* |
AF, atrial fibrillation; CI, confidence interval; IIR, image intensity ratio.
Values are expressed as percentage (95% confidence interval).
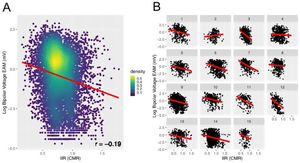
A point-by-point correlation between the EAM and IIR of the RA was evaluated in 15 patients undergoing a first AF ablation procedure. Overall, 11 404 voltage values were registered, and 8 830 (407 (324-560)) points per patient remained after exclusion those located>10mm apart from the CMR shell. A weak but significant negative correlation was found between the log-transformed bipolar voltage and the IIR (r = −0.19; P < .0001 in the correlation analysis; beta = −1.39; 95%CI, −1.54 to −1.23; P < .0001 in generalized linear mixed modeling) (figure 6).
Correlation between bipolar voltage and IIR in the 15 patients undergoing AF ablation. A: overall correlation in the pooled cohort. Rather than a simple linear regression, the analysis was performed with a generalized linear mixed model with random intercept to account for repeated IIR measurements per patient. B: analysis at the patient level. CMR, cardiac magnetic resonance; EAM, electroanatomical map; IIR, image intensity ratio.
Subsequently, each of the EAM- and IIR-paired points were labelled as healthy tissue, interstitial fibrosis or dense scar, and agreement between the 2 was tested (table 5). In comparison with EAM, LGE-CMR tended to underestimate RA fibrosis (healthy tissue CMR 81.0%; 95%CI, 80.2-81.8 vs EAM 60.6%; 95%CI, 59.6-61.6 for LGE-CMR and EAM, respectively). The overall agreement between the 2 techniques was 56%.
Consistency between techniques in fibrosis categorization
| EAM (bipolar voltage) | |||
|---|---|---|---|
| LGE-CMR (IIR) | Dense | Interstitial | Healthy |
| Dense | 216 | 211 | 399 |
| Interstitial | 180 | 213 | 458 |
| Healthy | 1068 | 1592 | 4493 |
EAM, electroanatomical map; IIR, image intensity ratio; LGE-CMR, late gadolinium enhancement-cardiac magnetic resonance.
Values are expressed as count (number of pixels). Agreement between pixels classified as healthy tissue, interstitial fibrosis or dense scar in both the electroanatomical map (EAM) and the LGE-CMR.
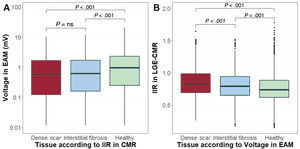
Then, bipolar voltage was averaged in each of the 3 LGE-CMR areas (eg, healthy tissue, interstitial fibrosis, dense scar). Bipolar voltage progressively increased from LGE-CMR-labelled areas as dense scar (mean EAM bipolar value 0.91mV; 95%CI, 0.52-1.31) to interstitial fibrosis (1.11mV; 95%CI, 0.72-1.50) to healthy tissue (1.77mV; 95%CI, 1.40-2.15; P<.0001). Similarly, IIR progressively decreased from EAM-labelled areas as dense scar (mean IIR 0.87; 95%CI, 0.82-0.91) to interstitial fibrosis (IIR 0.81; 95%CI, 0.76-0-86) to healthy tissue (IIR 0.76; 95%CI, 0.72-0.81; P < .0001) (figure 7).
Comparative box plot charts (median and interquartile range values). A: bipolar voltage (EAM) in areas classified as healthy tissue, interstitial fibrosis, and dense scar by IIR (CMR). B: IIR values (CMR) in areas classified as healthy tissue, interstitial fibrosis, and dense scar by EAM. CMR, cardiac magnetic resonance; EAM, electroanatomical map; IIR, image intensity ratio.
The left atria of the same 15 patients were used as a comparator for accuracy. In 15 479 EAM points (968 (700-1382) points per patient), we obtained a weak but significant negative correlation between the log-transformed bipolar voltage and the IIR (r = −0.17; P < .0001; beta = −1.52; 95%CI, −1.62 to −1.42; P<.0001), similar to results in the RA.
DISCUSSIONIn this study, we describe a standardized method to assess RA fibrosis by means of LGE-CMR. Our results show that: a) an IIR>1.21 characterizes myocardial fibrosis in the RA, while dense scar may be identified by an IIR>1.29; and b) the IIR shows a weak but significant association with bipolar voltage in the RA.
LGE-CMR thresholds for atrial fibrosis are similar in the left and the right atriaSeveral methods have been proposed to characterize LA fibrosis.1 The validated UTAH method2 relies on the bimodal (healthy vs fibrosis) distribution of LA pixel intensity, but largely depends on the choice of an expert-led threshold.14 The IIR was later designed to improve interindividual reproducibility.15 Subsequently, characterization of healthy volunteers and patients who had undergone PV isolation enabled standardized categorization into healthy atrial myocardium, interstitial fibrosis, and dense scar.13 We chose healthy volunteers at very low risk of AF (ie, aged less than 30 years) to minimize the degree of aging-induced interstitial fibrosis, thereby establishing a fibrosis threshold beyond which there is an increase in AF risk. Following an analogous approach, in the present study, we found that although IIR values characterizing fibrosis in the RA differed from those previously established for the LA, these differences were minor and, potentially, of little clinical relevance. Indeed, an IIR > 1.21 identified fibrosis in the RA, and an IIR > 1.29 differentiated interstitial fibrosis from dense scar, compared with an IIR > 1.20 and IIR > 1.32 for total fibrosis and dense scar in the LA, respectively. Structural, molecular, and functional differences between the left and right atria are evidenced by their distinct average IIR. However, despite these differences between the 2 atria,16 fibrosis might be assessed with LGE-CMR using similar thresholds in both chambers.
Interestingly, the correlation between EAM and CMR was statistically significant but of a weak intensity, similar to findings in the LA.17 At least partially, such a low correlation might result from technical inaccuracies and other factors accounting for a decreased correlation, such as atrial size.17 Of note, histological assessment is the only gold standard for atrial fibrosis assessment, but is not feasible in healthy individuals. The accuracy of atrial voltage to estimate atrial fibrosis is uncertain. It is likely that structural and functional data provided by CMR and EAM, respectively, yield complementary information on atrial remodeling. Finally, late gadolinium enhancement is found in fibrotic areas, but may also represent inflammation or portions of venous embryologic origin.
Right atrium fibrosis and remodeling in atrial fibrillation pathologyThe driving role of the LA in sustaining AF in most patients is widely acknowledged. However, the RA also undergoes marked changes, and CMR,12 computed tomography18 and EAM19 data suggest a similar remodeling intensity in both atria. In some cases, however, the RA might be particularly relevant to AF pathology and therapy. Some conditions superimpose an excessive pressure and volume overload in the RA.20 Among AF patients, those with obstructive sleep apnea show decreased conduction velocity, lower electrogram voltage and a higher complexity in the RA than those without sleep apnea; notwithstanding, electrophysiological remodeling is also evident in sleep apnea patients.9 In patients with atrial septal defects undergoing closure, RA dysfunction is a better predictor of incident AF than left atrial echocardiographic indices.21 This may also hold true in settings such as congenital heart disease or reentrant arrhythmias after cardiac surgery, among others.
Overall, a “right origin AF”, characterized by right atrial ectopia and a right-to-left dominant frequency gradient during AF, has been reported in some patients.22,23 “Right AF” is associated with a smaller PV, LA, and left appendage, but a larger right appendage.22 These data highlight the need for better characterization of the biatrial substrate in patients with AF. Our results will enable a more detailed, noninvasive characterization of RA fibrosis in patients with AF and, particularly, in those in which the RA could play a predominant pathophysiological role.
Clinical implications of right atrium fibrosis assessmentMyocardial fibrosis is a hallmark of AF pathology, and its characterization in daily clinical practice may have marked preventive, prognostic, and therapeutic implications. LGE-CMR has arisen as a potentially powerful tool to noninvasively assess atrial fibrosis, with most efforts focused on the LA. Unfortunately, data on an optimized method for RA fibrosis quantification has been lacking, so it remains underexplored.
The DECAAF trial proved that pre-existing LGE-CMR-detected LA fibrosis predicts postablation outcomes,2 but it is unknown whether characterization of RA fibrosis yields additional information. Extra-PV foci sustaining AF have been found in approximately 20% of patients with AF, and in up to 35% of those with permanent AF.10 Personalized ablation protocols targeting extra-PV fibrosis in the LA have been investigated in randomized clinical trials. Both the DECAAF II24 and ALICIA25 trials recently failed to show improved outcomes when fibrotic patches were targeted. However, ablation of non-PV foci arising from the superior and inferior vena cava, the crista terminalis, the foramen ovale and the coronary sinus ostium10 has shown to terminate AF in some patients. Dedicated studies are needed to determine whether targeting LGE-CMR-detected RA fibrotic patches will serve to personalize ablation procedures.
Finally, the role of LA fibrosis estimation to flag those individuals at high risk of incident AF is still speculative, although supported by small studies.26 If confirmed, patients with potentially “right AF” may benefit from RA fibrosis estimation. Testing for these potential applications warrant a dedicated, optimized, and refined methodology to quantify RA fibrosis; our findings are crucial for that purpose.
LimitationsSome limitations of our work should be acknowledged. Although blood pool normalization aims to compensate interindividual variability, other parameters may still account for significant variability in correlation analyses. Interelectrode distance, catheter disposition and mapping density might not be completely comparable among groups and could modify the results. Technical inaccuracies should not be disregarded. Registration errors in the EAM due to pressure on the atrial wall, or cardiac and respiratory movements may yield subtle changes in catheter position that could have a strong impact on correlation analyses. Correlation analyses were performed in a point-by-point basis in 3D shells; alternative flattening methods27 or reducing the EAM-to-CMR tolerance distance (ie, 10mm in our study) may yield more accurate correlations.17
The external reproducibility of our thresholds is critical to ensure wide clinical application of RA fibrosis assessment. We used a 3.0T CMR setup that yields a high signal-to-noise ratio and improves image resolution. LA thresholds previously derived from 3.0T Images 13 have been recently validated in 1.5T setups; 28whether RA thresholds may be applied in 1.5T CMR setups needs to be proved. Similarly, images were obtained 20minutes after gadolinium administration to reach enough image contrast; 29shorter delays may result in different thresholds. Finally, our RA thresholds were obtained under specific parameters (see methods in the supplementary data) commonly used for LA fibrosis assessment, enabling the use of a single sequence for LA and RA fibrosis assessment; validity under different parameters cannot be ensured. We have recently shown good intra- and interobserver reproducibility even in the hands of inexperienced operators.30
CONCLUSIONSThe IIR threshold of the RA to determine healthy/fibrotic tissue was established at 1.21, close to the value used for the LA. Fibrosis quantification with CMR-LGE is feasible, and could be useful in both atria.
FUNDINGThis work was supported in part by a grant from the European Union Horizon 2020 Research and Innovation Programme under grant agreement [No 633196] (CATCH ME project); Instituto de Salud Carlos III [PI16/00435, PI19/00573]; Agència de Gestió d’Ajuts Universitaris i de Recerca [AGAUR, 2017 SGR 1548]; Fundació la Marató de TV3 [20152730]; CERCA Programme/Generalitat de Catalunya. The sponsor was not involved in the study design, data collection, analysis or interpretation of the results, the writing of the manuscript, or the decision to publish the work.
AUTHORS’ CONTRIBUTIONSC. Gunturiz-Beltrán: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, original draft writing, review of the draft and editing, visualization. R. Borràs: methodology, software, formal analysis. F. Alarcón, P. Garre, R. M. Figueras i Ventura: methodology, software. E.M Benito: conceptualization, methodology. G. Caixal, T.F, Althoff, J.M. Tolosana, E. Arbelo, I. Roca, J. Brugada: conceptualization, visualization. S. Prat-Gonzalez, R.J. Perea: software, visualization. M. Sitges: conceptualization, visualization, validation. E. Guasch: conceptualization, methodology, validation, formal analysis, investigation, resources, review of draft and editing, visualization, supervision. L. Mont: conceptualization, methodology, validation, resources, review of drafts and editing, supervision, project administration, funding acquisition. E. Guasch and L. Mont share senior authorship.
Conflicts of InterestL. Mont has received research grants, support for a fellowship program, and honoraria as a consultant and lecturer from Abbott, Boston Scientific, Medtronic, and Biosense, and is a shareholder for Galgo Medical S.L. M. Sitges has received research grants and honoraria as a consultant and speaker from Abbott, Medtronic, and Edwards Lifesciences. The remaining authors declare no conflicts of interest.
- -
Atrial fibrosis is a determinant in the pathogenesis of AF as part of structural remodeling.
- -
LGE-CMR allows noninvasive characterization of atrial fibrosis and could be used to personalize AF ablation.
- -
Research has been focused on determining fibrosis in the LA, but no study has previously assessed right atrial fibrosis with magnetic resonance. This limits our ability to comprehensively characterize its contribution to AF pathology.
- -
Thresholds identifying right atrial fibrosis in LGE-CMR: the IIR values above 1.21 identify total atrial fibrosis (interstitial fibrosis and dense scar, if present), and IIR values above 1.29 discern dense scar from interstitial fibrosis.
- -
IIR values to localize myocardial fibrosis are similar in the LA and RA.
- -
Endocardial bipolar voltage correlates with IIR in the RA.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.06.010