Changes in sex hormone levels are a known triggering factor for spontaneous coronary artery dissection (SCAD) in women. However, it is unknown whether exposure to exogenous hormone therapy (HT) at the time of SCAD presentation modifies the clinical course of this condition. We investigated the association between HT in female patients presenting with SCAD and short-term clinical outcomes.
MethodsWe enrolled consecutive patients presenting with SCAD from the DISCO-IT/SPA (dissezioni spontanee coronariche Italian-Spanish) registry. Women on HT (estrogens, progestagens, or gonadotropins) at the time of presentation were identified, and their clinical characteristics and short-term outcomes were compared with those not receiving active HT. The outcome measure was nonfatal myocardial infarction and/or unplanned percutaneous coronary intervention during the first 28 days after the index catheterization.
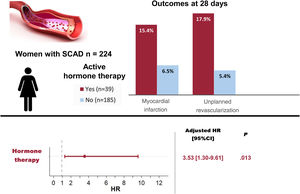
ResultsOf 224 women presenting with SCAD (mean age 52.0±10.0 years), 39 (17.4%) were currently using HT while 185 (82.6%) were not. No significant differences were noted in the baseline demographics, clinical presentation, angiographic features, or initial treatment received between the 2 groups. All patients on systemic HT (n=36, 92%) discontinued it at the time of diagnosis. The composite outcome occurred in 7 (17.9%) patients with prior HT compared with 14 (7.6%) without (P=.039). After multivariable adjustment, HT remained associated with the composite outcome recorded in the first 28 days of follow-up (HR, 3.53; 95%CI, 1.30-9.61; P=.013).
ConclusionsIn women with SCAD, exposure to HT at the time of clinical presentation was associated with short-term recurrent cardiovascular events such as nonfatal myocardial infarction and/or unplanned percutaneous revascularization.
Keywords
Spontaneous coronary artery dissection (SCAD) is a cause of acute coronary syndrome that has a clear female preponderance (81%-92%).1 Its reported incidence has increased over recent years, in part due to growing awareness of this condition and improved diagnosis with imaging techniques.2 Recent studies suggest that SCAD is the fundamental cause of as many as 35% of all myocardial infarctions in women <50 years of age, and the most common etiology of pregnancy-associated myocardial infarction (MI).3
Despite recent advances in the understanding of SCAD pathology, the mechanisms underlying the etiology remain largely unclear.4 Hypotheses encompassing the potential role of sex hormones in SCAD are supported by the clear female preponderance of the disease and its relationship with pregnancy.5–7 Causal links involve hormone-induced connective tissue changes, including loss of elastic fiber structure, collagen degeneration, smooth muscle hypertrophy, shearing stress, and altered mucopolysaccharide and protein composition of the arterial media.8,9
Consequently, the involvement of exogenous hormone therapy (HT) in SCAD and the subsequent clinical course of patients on these therapies remains a matter of debate.10,11 To date, data on the impact of HT prior, during or following a SCAD event is scarce.12,13 In this study, we aimed to investigate whether prior exposure to HT in female patients with SCAD influenced subsequent short-term clinical outcomes in terms of nonfatal MI and/or unplanned percutaneous revascularization.
METHODSStudy design and populationDISCO-IT/SPA (dissezioni spontanee coronariche Italian-Spanish) is an observational, international, multicenter, retrospective registry which enrolled SCAD patients from 26 centers. Patients were enrolled in the registry from 1 January 2009 to 31 December 2019. For the present analysis, we included women with clinical presentation compatible with acute coronary syndrome and angiographic coronary features meeting the criteria for SCAD.6 All cases were confirmed and classified angiographically by a core laboratory, as previously described.14,15 We excluded patients with significant (≥ 50%) atherosclerotic disease in other coronary arterial segments or with an underlying complicated plaque revealed by intracoronary imaging. We designed a dedicated electronic case report form16 and an informed consent form.
Demographic data including exposure to HT, clinical presentation, angiographic findings, management, and outcomes were extracted from clinical source documents or were collected via medical records, patient interviews, and follow-up visits. Treatment with HT was assessed via direct patient interview and/or by checking the electronic prescription system, but not necessarily both. HT could include estrogens, progestogens or gonadotropins and the patient had to have been on it at the time of the SCAD event, with an undefined onset time. The clinical indications for these treatments encompassed contraception, hormone replacement therapy for climacteric symptoms or infertility. Pregnancy-associated SCAD was defined as the presentation of the index event during pregnancy or within 12 months of delivery.17 A dedicated data manager (L. Lo Salvio) oversaw source verification, quality control, and queries from the coordinating center to the participating sites to minimize bias. The study was approved by the institutional review committees and was conducted in accordance with the Declaration of Helsinki.
Follow-up, and outcomesAdverse events were reported in a specific section of the eCRF. Coronary angiograms as well as available clinical information (clinical presentation, 12-lead electrocardiogram, troponin I values) were checked by the coordinating center to adjudicate the event. Clinical outcomes included all-cause death, nonfatal MI (fourth universal definition of MI18), any unplanned revascularization, stroke, or Bleeding Academic Research Consortium (BARC) bleeding events. Percutaneous coronary intervention (PCI) success was defined as Thrombolysis in Myocardial Infarction (TIMI) flow 2-3 with residual stenosis <30% (after stent/scaffold implantation) or <50% (after simple balloon angioplasty) in the first catheterization. The primary composite outcome was nonfatal MI (fourth universal definition of MI)18 and/or unplanned PCI at any point after the index catheterization and until 28 days of follow-up (4 weeks). Given that HT was discontinued in most patients on this therapy, we decided to analyze the primary outcome at 28 days to examine a more direct relationship with the exposure. The 12-month outcomes are also reported.
Statistical analysisThe grouping variable was HT (2 groups). Noncategorical variables are summarized using means and were compared using ANOVA or Kruskal-Wallis tests according to the normality of distributions. Categorical variables are expressed as percentages and were compared using the chi-square or Fisher exact tests, if required. Kaplan-Meier curves were plotted for the time to occurrence of the composite outcome by each group and were compared using the log-rank test. Multivariable adjustment was conducted using Cox regression including age and covariables with a significance level below 0.20 in the univariate analysis along with those considered clinically relevant.
In a complementary analysis, the inverse probability of treatment weighting (IPTW), a propensity score method, was used to adjust baseline variables that were distributed differently between groups. This was followed by multivariable Cox regression to control by known or potential confounders related to the outcome (STEMI, multivessel disease, angiotype 2A+3, TIMI flow 3, dual antiplatelet therapy). The IPTW was estimated using a logistic regression model that included all potential confounders that were distributed differently between groups (age, smoking status, hypertension, dyslipidemia, migraines). We then performed a balance assessment, comparing the distribution of measured baseline covariables between groups using standardized differences before (raw) and after (weighted) the IPTW. As a rule of thumb, a standardized difference of <0.10 may be considered a negligible imbalance between groups.19 Additionally, we used the overidentification test for covariate balance to assess the result of the IPTW. Statistical significance was established at P ≤ .05 (2-tailed) for the comparisons and measures of association. All statistical analyses were conducted using Stata IC 15.1 (Stata Corp, College Station, United States).
RESULTSA total of 302 patients were enrolled in the registry; 267 (88.4%) were women. Of these, 43 (15.4%) were excluded because of missing key data (HT) or insufficient minimum follow-up. These patients did not differ from those with complete data (table 1 of the supplementary data). Thus, 224 women with complete data and follow-up at a minimum of 28 days were included in this study. Overall, mean age was 52.0±10.0 [range 29-84] years. A total of 39 patients (17.4%) were receiving HT for a median time of 3 years (IQR: [1-7]), which in most cases was oral contraception (51.3%, table 1). Only the 3 patients with an intrauterine device continued their prescription following their events, at least within the first month that was recorded here.
Type and duration of hormone therapy at the time of spontaneous coronary artery dissection
| Type | Number (n=39) | Duration |
|---|---|---|
| Hormone replacement therapy (combined) | 12 (30.8) | 3.8±4.6 |
| Oral combined contraception | 20 (51.3) | 7.5±7.8 |
| Oral progestagen contraception | 2 (5.1) | 3±2 |
| Topical progestagen (IUD) | 3 (7.7) | 1.7±1.2 |
| Fertility (gonadotropines, estrogen and progestagen) | 2 (5.1) | 1.1±1.2 |
IUD, intrauterine device.
Data are expressed as No. (%) or mean±standard deviation.
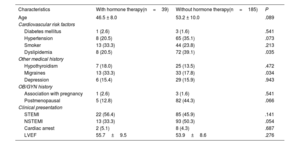
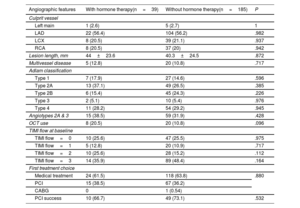
Baseline characteristics of patients with and without HT at the time of the event were similar, except for a higher prevalence of migraines and a lower prevalence of dyslipidemia in the HT group (table 2). In terms of clinical presentation, 47.8% presented with ST-elevation MI (STEMI) and 47.3% with non–ST-elevation MI (NSTEMI). The main angiographic features of both groups, including SCAD angiotype, did not differ significantly (table 3). Most patients (n=142, 63.4%) were managed conservatively as the initial strategy, without significant differences among groups. Moreover, 82 patients underwent ad hoc PCI (36.6%), of which 72.0% were considered successful by the core laboratory analysis. One patient (0.5%) underwent coronary artery bypass graft surgery as the first therapeutic option. The medications administered, including antiplatelet therapy, were not different between the 2 groups (table 4). Median hospital stay was 6 (IQR [5-8]) days. At the time of discharge, a total of 101 patients (45.1%) had undergone revascularization (n=100 PCI and n=1 coronary artery bypass graft). There were no deaths.
Baseline clinical characteristics and clinical presentation
| Characteristics | With hormone therapy(n=39) | Without hormone therapy(n=185) | P |
|---|---|---|---|
| Age | 46.5 ± 8.0 | 53.2 ± 10.0 | .089 |
| Cardiovascular risk factors | |||
| Diabetes mellitus | 1 (2.6) | 3 (1.6) | .541 |
| Hypertension | 8 (20.5) | 65 (35.1) | .073 |
| Smoker | 13 (33.3) | 44 (23.8) | .213 |
| Dyslipidemia | 8 (20.5) | 72 (39.1) | .035 |
| Other medical history | |||
| Hypothyroidism | 7 (18.0) | 25 (13.5) | .472 |
| Migraines | 13 (33.3) | 33 (17.8) | .034 |
| Depression | 6 (15.4) | 29 (15.9) | .943 |
| OB/GYN history | |||
| Association with pregnancy | 1 (2.6) | 3 (1.6) | .541 |
| Postmenopausal | 5 (12.8) | 82 (44.3) | .066 |
| Clinical presentation | |||
| STEMI | 22 (56.4) | 85 (45.9) | .141 |
| NSTEMI | 13 (33.3) | 93 (50.3) | .054 |
| Cardiac arrest | 2 (5.1) | 8 (4.3) | .687 |
| LVEF | 55.7±9.5 | 53.9±8.6 | .276 |
LVEF, left ventricular ejection fraction, NSTEMI, non–ST-segment elevation myocardial infarction; OB/GYN: obstetrics and gynecology; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as No. (%) or mean±standard deviation.
Angiographic features, and first treatment choice
| Angiographic features | With hormone therapy(n=39) | Without hormone therapy(n=185) | P |
|---|---|---|---|
| Culprit vessel | |||
| Left main | 1 (2.6) | 5 (2.7) | 1 |
| LAD | 22 (56.4) | 104 (56.2) | .982 |
| LCX | 8 (20.5) | 39 (21.1) | .937 |
| RCA | 8 (20.5) | 37 (20) | .942 |
| Lesion length, mm | 44±23.6 | 40.3±24.5 | .872 |
| Multivessel disease | 5 (12.8) | 20 (10.8) | .717 |
| Adlam classification | |||
| Type 1 | 7 (17.9) | 27 (14.6) | .596 |
| Type 2A | 13 (37.1) | 49 (26.5) | .385 |
| Type 2B | 6 (15.4) | 45 (24.3) | .226 |
| Type 3 | 2 (5.1) | 10 (5.4) | .976 |
| Type 4 | 11 (28.2) | 54 (29.2) | .945 |
| Angiotypes 2A & 3 | 15 (38.5) | 59 (31.9) | .428 |
| OCT use | 8 (20.5) | 20 (10.8) | .096 |
| TIMI flow at baseline | |||
| TIMI flow=0 | 10 (25.6) | 47 (25.5) | .975 |
| TIMI flow=1 | 5 (12.8) | 20 (10.9) | .717 |
| TIMI flow=2 | 10 (25.6) | 28 (15.2) | .112 |
| TIMI flow=3 | 14 (35.9) | 89 (48.4) | .164 |
| First treatment choice | |||
| Medical treatment | 24 (61.5) | 118 (63.8) | .880 |
| PCI | 15 (38.5) | 67 (36.2) | |
| CABG | 0 | 1 (0.54) | |
| PCI success | 10 (66.7) | 49 (73.1) | .532 |
LAD, left anterior descending artery; LCX, left circumflex; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, thrombolysis in myocardial Infarction.
Data are expressed as No. (%) or mean±standard deviation.
Pharmacological treatment received
| Drug | With hormone therapy(n=39) | Without hormone therapy(n=185) | P |
|---|---|---|---|
| UFH | 31 (79.5) | 142 (76.8) | .545 |
| GpIIb/IIIa | 2 (5.1) | 15 (8.1) | .227 |
| Antiplatelet monotherapy | 11 (28.2) | 46 (24.9) | .687 |
| Dual antiplatelet therapy | 28 (71.8) | 139 (75.1) | .707 |
| Beta-blockers | 29 (74.4) | 156 (84.3) | .430 |
| Calcium antagonist | 4 (10.3) | 18 (9.7) | .526 |
UFH; unfractionated heparin; GpIIb/IIIa, glycoprotein-IIb/IIIa inhibitors.
Data are expressed as No. (%).
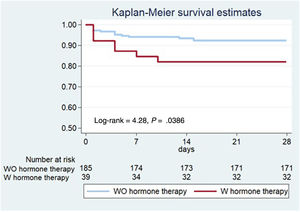
The primary composite outcome following the index catheterization and during the first 28 days of follow-up, occurred in a higher proportion in the HT group: 7 (17.9%) vs 14 (7.6%) patients, log-rank=4.28, P=.0386 (figure 1, table 5). The 7 patients with events in the HT group were on oral contraception (n=4) or hormone replacement therapy (n=3), and none had a recent/current pregnancy. More patients on HT required unplanned PCI: 7 (17.9%) vs 10 (5.4%), P=.007; and the most common indication was chest pain with evidence of ischemia on electrocardiogram (87.5%). Of those requiring unplanned PCI, most (n=13, 72.2%) experienced clear progression of the initial dissection with worsening of angiographic flow. Furthermore, a trend toward a higher incidence of MI in the HT group was observed (15,4% vs 6.5%, P=.063). The differences observed in clinical outcomes did not appear to be influenced by having received either PCI or conservative management (table 5). After multivariable adjustment in Cox regression analysis, HT remained significantly associated with the composite outcome: adjusted HR, 3.53; 95%CI, 1.30-9.61, P=.013 (table 2 of the supplementary data). Consistently, the IPTW followed by another multivariable Cox regression yielded an adjusted HR of 3.65; 95%CI, 1.51-8.80; P=.004 (table 3 of the supplementary data).
Clinical outcomes at 28-days and 12-months of follow-up
| With hormone therapy(n=39) | Without hormone therapy(n=185) | P | |
|---|---|---|---|
| 28-days adverse events | |||
| Composite outcome | 7 (17.9) | 14 (7.6) | .043 |
| Nonfatal myocardial infarction | 6 (15.4) | 12 (6.5) | .063 |
| Unplanned PCI | 7 (17.9) | 10 (5.4) | .007 |
| Deaths | 0 | 0 | - |
| Stroke or TIA | 0 | 1 (0.5) | .914 |
| Bleeding BARC type 1 or 2 | 1 (2.6) | 3 (1.6) | .541 |
| 28-days composite outcome by first treatment chosen | |||
| Medical treatment (n=142) | 4/24 (16.7) | 8/118 (6.8) | .121 |
| PCI (n=82) | 3/15 (20) | 6/67 (8.9) | .353 |
| 12-month adverse events | |||
| Composite outcome | 8 (20.5) | 20 (10.8) | .095 |
| Nonfatal myocardial infarction | 6 (15.4) | 15 (8.1) | .156 |
| Unplanned PCI | 8 (20.5) | 13 (7.0) | .008 |
| Deaths | 0 | 0 | - |
| Stroke or TIA | 0 | 1 (0.5) | .914 |
| Bleeding BARC type 1 or 2 | 1 (2.6) | 3 (1.6) | .541 |
PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; BARC, Bleeding Academic Research Consortium.
Data are expressed as No. (%).
The 12-month outcomes are also shown in table 5. Although the absolute difference in the composite outcome remained large between patients with and without HT, it was no longer statistically significant (20.5% vs 10.8%, P=.095). The incidence of nonfatal MI was also not statistically different (15.4% vs 8.1%, P=.156). In contrast, the difference in unplanned PCI remained significant (20.5% vs 7.0%, P=.008). No deaths were recorded during the first year of follow-up in the study population.
DISCUSSIONIn our multicenter SCAD registry, being on HT at the time of the SCAD index event was associated with an increased risk of short-term nonfatal MI and/or unplanned percutaneous revascularization (figure 2). This finding can be helpful in identifying patients at higher risk of early recurrent events, who may benefit from close surveillance following SCAD.
Central illustration. Women on active hormone therapy at the time of presenting spontaneous coronary artery dissection (SCAD) showed an increased rate of events at 28 days. Hormone therapy was an independent predictor of major cardiovascular events (MACE). HR adjusted by age, smoking status, multivessel disease, angiotypes 2A and 3 and double antiplatelet therapy; 95%CI, 95% confidence interval; HR, hazard ratio.
Female exogenous hormones have various effects in the cardiovascular system. From a clinical viewpoint, hormone replacement therapy and some forms of oral contraception have been shown to increase the risk of venous thromboembolism and stroke in the healthy population, whereas only the latter has been found to pose a higher risk of MI.20,21 The effects of estrogen on arteries vary with the stage of reproductive life, being protective against the development of atherosclerosis in premenopausal women.22 On the other hand, we know that patients on HT (estrogen and progestogen) may experience subtle alterations of the arterial wall caused by fragmentation of the reticulin fibers, degeneration of collagen, loss of normal corrugation of elastic fibers, hypertrophy of the smooth muscle cells and changes in the mucopolysaccharide content and protein composition of the media. All these factors contribute to a weakening of the latter and ultimately to dissection.8,23 Moreover, female hormones contribute to fluid retention and reduction of peripheral resistance, resulting in increased cardiac output and thus potentially facilitating hemorrhage into the media and intimal rupture.24
The modification in vessel wall structure associated with HT may increase vessel frailty in patients who already have a propensity to develop SCAD, potentially acting as a trigger for the acute SCAD event and/or as a prognostic factor for clinical progression requiring urgent revascularization. In this regard, Antonutti et al.12 previously reported an association of HT with recurrent de novo SCAD in long-term follow-up but lacked statistical power (n=60 females) to confirm this in multivariable analyses. In a prospective cohort of patients with nonatherosclerotic SCAD from 22 centers in North America (n=750), active HT was not found to be associated with worse outcomes (10% of the patients enrolled). However, their study population differed from ours as they had a lower risk profile, characterized by a lower proportion of STEMI and PCI.25 In a cohort study using the Mayo Clinic SCAD “Virtual” Multi-Center Registry (n=563), Kok et al.26 reported that 17% of the female patients were on exogenous hormones. Our study, with a similar prevalence of the use of these therapies, shows that prior exposure to HT was significantly associated with higher rates of reinfarction and/or unplanned percutaneous coronary revascularization during short-term follow-up. At 12 months of follow-up, the difference was no longer statistically significant but remained clinically relevant (20.5% vs 10.8%). The limited statistical power of the study mandates confirmation of its findings in larger and prospective cohorts.
Hormone status in women varies throughout the lifespan. In our study, we focused on exposure to HT and its short-term implications after SCAD diagnosis, regardless of age and hormone status. A specific analysis of the effect of these exogenous treatments in SCAD according to patients’ baseline hormone status is pertinent. Unfortunately, we lacked the power to obtain the data, but we are keen to seek it in the future. Menopausal status was not associated with outcomes in our study. Similarly, Díez-Villanueva et al.27 showed that postmenopausal women with SCAD had similar in-hospital outcomes compared with premenopausal women, although they had different clinical and angiographic characteristics. On the other hand, Saw et al.5 found that peripartum SCAD was significantly associated with 30-day MACE. This observation, alongside other studies revealing the poorer outcomes of pregnancy-associated SCADsupport the link between hormone shifts and a worse short-term prognosis in patients with SCAD. In this regard, all women using systemic HT in our study had their treatment discontinued following admission, which could have influenced the development of short-term adverse events.
When studying the role of hormones in SCAD pathophysiology, a potential link with vascular endothelial dysfunction may also be considered. We previously reported that SCAD patients have a worse endothelial function compared with matched controls.28 However, data on endothelial function in patients treated with HT is heterogeneous due to the wide variety of treatment modalities and forms available. While hormone replacement therapy seems not to alter endothelial function,29 oral contraceptive pills have shown mixed results depending on doses and combination forms.30 Unfortunately, vascular endothelial function was not systematically assessed in our registry, hence we cannot explore this potential association. A dedicated study to evaluate the interaction between endothelial function and HT in SCAD patients is warranted.
Furthermore, a history of migraine was more prevalent in patients on HT of our cohort, whereas dyslipidemia was less frequent in this group. Patients on HT tended to be younger (46.5 vs 53.2 years old; P=.089) and premenopausal (87% vs 56%; P=.066), which could potentially explain the higher prevalence of migraines and lower propensity to have dyslipidemia. Additionally, HT is sometimes prescribed for migraine attacks, which improves blood lipid control as a side effect.31 According to Kok et al., migraine could be more common among SCAD patients than in the normal population, which could reflect an underlying propensity to vascular damage in these patients. However, apart from more often experiencing chest pain in the first month, SCAD patients with migraine did not show a different prognosis.26 Similarly, we did not find an association between migraine and adverse clinical outcomes in our study. Moreover, the complementary analysis performed with the IPTW method was adjusted by all these baseline variables.
Study limitationsThe findings of this study may be affected by several limitations inherent to its retrospective nature. Despite the implementation of a multivariable model and the IPTW, we cannot exclude the influence of unknown confounders. Likewise, given the study design, which only included patients who had developed and survived the disease, potential selection bias (including collider bias) cannot be excluded. Moreover, the limited statistical power impedes exclusion of type 1 and 2 errors and precluded the performance of a more detailed analysis of the type and duration of HT and its relationship with outcomes. In fact, studying subpopulations of an already infrequent medical condition often results in limited sample sizes and statistical power, which hampers the drawing of robust conclusions on the findings. HT status was not collected in a proportion of patients (16.1%) who were consequently excluded, showing no gross differences with those included (table 1 of the supplementary data). The exposure studied gathered distinct types of HT, with potential different clinical implications. The continuation of HT following the event and during long-term follow-up was not assessed and therefore we could not objectively evaluate the impact of these therapies on long-term recurrent events.
CONCLUSIONSThe present observational study shows that women with SCAD and previous exposure to HT may have a higher risk of nonfatal MI and/or unplanned coronary revascularization after index catheterization and during the first 28 days of follow-up. Our findings, along with those of other studies, will help to depict a high-risk profile for patients with SCAD who may merit longer admission with closer surveillance. The precise role of HT and endogenous hormone shifts in the pathogenesis of SCAD remains to be elucidated.
FUNDINGR. Mori received an educational grant from the European Society of Cardiology (APP000019660). This was an investigator-initiated research project. Only the electronic database of the registry is financed with a grant from the Fundación Interhospitalaria de Investigación Cardiovascular.
AUTHORS’ CONTRIBUTIONSR. Mori and F. Macaya contributed equally to this work. R. Mori: data collection, formal analysis, writing original draft. F Macaya: conceptualization, data collection, methodology, supervision, writing original draft, critical review. F. Giacobbe: data collection, methodology, formal analysis, review and editing. V. Moreno, G. Quadri, D. Chipayo, M. Bianco, P. Salinas, C. Rolfo, H. Mejía-Rentería, A. Boi, G. Tirado Conte, Ch. Cavallino, L. Nombela, S. Cinconze, P. Jiménez-Quevedo, M. Pavani, A. Chinaglia and I.J. Nuñez Gil: data collection, review and editing. M.E. Fuentes-Ferrer: formal analysis, methodology, supervision. E. Cerrato and N. Gonzalo: data collection, methodology, supervision, key critical review. A. Fernandez-Ortiz, F. Varbella and J. Escaned: Resources, supervision, review and editing.
CONFLICTS OF INTERESTThere are no conflicts of interest to declare relevant to this paper.
- -
SCAD has a female preponderance and is an important cause of MI in young women and in the puerperium. Female hormones are presumed to play a role in the development of the disease. The impact of exogenous HT in patients with SCAD is unknown.
- -
In this multicenter registry, HT was associated with higher rates of short-term major cardiovascular events such as nonfatal MI and/or unplanned percutaneous revascularization. Being on active HT at the time of presenting SCAD may pose a higher risk of early recurrent events.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.07.004