Keywords
INTRODUCTION
Pulmonary thromboembolism (PE) hasremained a "no-man's-land" for decades. It waspositioned somewhere between pulmonology andcardiology, internal medicine and intensive care,radiology and nuclear medicine, hematology andcardio-thoracic surgery. Moreover, the strongestrisk factors for PE cluster in patients followedin orthopedic, neurological, oncological, andobstetric departments. Physicians faced with PEare speaking different languages and using different management strategies depending on their clinicalbackground. There seems to be a chance to put anend to PE Babel Tower. This is due to the growingunderstanding that the key clinical problems—both in acute PE and chronic thromboembolicpulmonary hypertension (CTEPH)—are relatedto right ventricular (RV) pressure overload andfailure. Cardiologists, including intensive cardiaccare specialists, are therefore becoming moreinterested in understanding and combating thedisease. European Society of Cardiology was thefirst to elaborate comprehensive clinical practiceguidelines in PE and CTEPH.1,2 Importantly,Task Forces preparing and updating thedocuments included radiologists, pulmonologists,hematologists, intensivists and surgeons, offeringa universally acceptable common platform forcommunication. The current text will respect therecent European Society of Cardiology guidelines,adding new evidence wherever necessary.
ACUTE THROMBOEMBOLIC DISEASE
Epidemiology and Predisposing Factors
The epidemiology of PE is difficult to estimatebecause of non-specific presentation and commonerrors in diagnosis. The annual incidence rateof venous thromboembolism (VTE) is probablybetween 20 and 70 cases per 100 000 population.3,4Approximately one third of those patients will haveacute PE while the remaining will have isolated deepvein thrombosis (DVT).5 Clinical and post-mortemdata collected in Malmo area in Sweden, wheremost deaths were followed by autopsy, suggestedthe incidence of PE of approximately 20/10 000inhabitants/year.6 Approximately 10% of all patientswith acute PE die during the first 1-3 months.7,8 One of each 10 patients dying in the hospital willdie because of PE and one out of each 100 patientsadmitted to the hospital will die because of it.9-11
Venous thromboembolism is a result of theinteraction between patient-related and setting-related risk factors. Patient-related predisposingfactors are usually permanent while setting-relatedpredisposing factors are temporary12 (Table 1). PEwhich occurs in the absence of any obvious settingrelated factor is often called "unprovoked."
Patient-related predisposing factors include age,history of previous VTE, active cancer, neurologicdisease with extremity paresis, medical disorderscausing prolonged bed rest, such as heart orrespiratory failure, and congenital or acquiredthrombophilia, hormone replacement therapy, oralcontraceptive therapy.12 The 2 last factors can bealso considered as setting related, particularly, ifan embolic episode occurs relatively early after thebeginning of hormonal administration. Identificationof the presence and estimation of relative significanceof predisposing factors may be helpful both inassessment of clinical probability for diagnosticpurposes as well as for decisions regarding primaryprevention. Unfortunately, PE can occur in patientswithout any identifiable predisposing factors. Theproportion of patients with idiopathic or unprovoked PE was about 20% in the International CooperativePulmonary Embolism Registry (ICOPER).13 In several areas of particular interest or controversy,new relevant evidence related to predisposing factorsemerged recently:
- A meta-analysis of 14 studies assessed travel as aVTE predisposing factor. Based on 4055 documentedVTE cases, its relative risk in travelers was increasedby 2.8 (CI, 2.2-3.7). Risk increased by 18% and 26%for each 2-hour increase in duration of travel by anymode and by airplane, respectively.14
- In a Danish national cohort study with 10.4 million woman years recorded, including 3.3 million woman years in receipt of oralcontraceptives, 4,213 venous thrombotic events,including PE, were observed. The overall absoluterisk of VTE per 10 000 woman years was 3.01in non-users and 6.29 in current users of oralcontraceptives. The risk decreased with durationof use and decreasing oestrogen dose. Oralcontraceptives with desogestrel, gestodene, ordrospirenone were associated with a 1.5 to 2.0higher risk than with levonorgestrel. Progestogenonly pills and hormone releasing intrauterinedevices were not associatd with increased risk ofvenous thrombosis.15
- To clarify which obesity marker best describesincreased VTE risk 27 178 men and 29 876 women 50to 64 years of age were followed in prospective studyfor 10 years. Six hundred forty one VTE incidentswere verified by review of medical records. Hipcircumference was positively associated with VTE inwomen but not in men, whereas waist circumferencewas positively associated with VTE in men but notin women. Positive associations were found betweenVTE and body weight, body mass index and totalbody fat mass.16
The mystery of idiopathic PE remains unexplored.Recently, markers of inflammation, such as highsensitivity-C Reactive Protein (hs-CRP), fibrinogen,and factor VIII, were found to be increased inpatients with idiopathic compared to "secondary" VTE,17 supporting the hypothesis that the formermay share some predisposing factors with arterialthromboembolism.18
The genetic background of VTE was highlitedby a recent finding that thrombosis at a young agein patients was the strongest predictor of venousthromboembolism in relatives, (OR, 3.27; 95% CI, 1.68-6.38) for patients <45 years of age at the timeof VTE compared with those >71 years of age.Interestingly, the presence of factor V Leiden orthe G20210A thromboplastin gene was a weakerindependent predictor of venous thromboembolismin relatives (adjusted OR, 1.48; 95% CI, 0.94-2.33).19 Genetic contribution to the aetiology of venous thromboembolism has been recently assessed inmore detail in a meta-analyses which included126 525 cases and 184 068 controls derived from 173case-control studies.20 It looked at 21 genes and 28polymorphisms. In Caucasian population FactorV G1691A and A4070G, prothrombin G20210Aand G11991A, PAI-1 4G/5G, alpha-fibrinogenThr312Ala were found to be significantly associatedwith VTE. Interestingly, Factor XIII Val34Leu andbeta-fibrinogen 455 G/A both showed significantlyprotective effects.
While all these data improve our understanding ofthe VTE pathophysiology and particularly supportthe concept of an important genetic component inthe aetiology of idiopathic VTE disease, they donot offer at present much help in everyday clinicalmanagement.
Diagnostic Management According to InitialPrognostic Staging
Current approach to a patient with suspectedPE is based on initial prognostic stratificationinto patients at high (>15%) and non-high risk ofearly PE-related death.1 This stratification is basedentirely on clinical evaluation, namely a search forthe presence of shock or systemic hypotension.Hypotension is defined as systolic blood pressure <90 or its fall by ≥40 mmHg compared to usuallevel for at least 15 min and without an apparentalternative cause.21
High-Risk Patients
Most of the diagnostic recommendations regardingpatients in shock or hypotension do not have supportin evidence from appropriately designed trails andare based on expert opinions.
In high-risk patients the priority is puton emergency confirmation or exclusion ofhemodynamically significant pulmonary arterialthrombi, which are usually multiple, large andproximal. However, first line diagnostic testshould also allow differential diagnosis with otherimmediately life threatening conditions. Acutecoronary syndromes, aortic dissection with cardiactamponade, acute left ventricular or valvulardysfunction but also tension pneumothorax oreven major internal bleeding may all present withsymptoms and signs to similar those occurringin acute PE, including acute dyspnea, chest pain,syncope, hemodynamic instability.
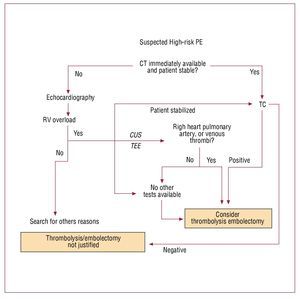
Computed tomography (CT) angiography is therecommended first-choice diagnostic test in highrisk patients suspected of PE (Figure 1). However,bedside emergency echocardiography is anacceptable alternative in case clinical condition of the patient is critical.1 Normal RV echocardiogramexcludes life-threatening PE. Unequivocal signs ofacute RV pressure overload are highly suggestive(though not diagnostic) of PE in such clinicalsetting. Intravenous unfractionated heparin (UFH)should be started and thrombolytic therapy shouldbe strongly considered.1 Right heart thrombi foundat echocardiography justify aggressive treatmentincluding thrombolysis or embolectomy, withoutthe need of further diagnostic evaluation.22
Figure 1. Suggested diagnostic algorithm in patients with suspected acute pulmonary thromboembolism presenting with shock or hypotension. CT indicates computed tomography angiography; CUS, venous compression ultrasound; TEE, transesophageal echocardiography; RV, right ventricle; PE, pulmonary thromboembolism. Italics and dotted lines represent additional suggestions, not included in the original European Society of Cardiology guidelines algorithm.
While preparing for thrombolytic therapy,confirmation with CT (in case the patient could bestabilized) or bedside venous compression ultrasound(CUS) or transesophageal echocardiography (TEE)should be considered.23 Direct confirmation ofthrombi is particularly important in patients withrelative contraindications to thrombolysis. Noninvasive assessment is preferable, in view of lowerrate of local bleeding complications.24,25 However, classical angiography can also be useful, particularlyin a patient referred to hemodynamic laboratorybecause of suspected acute coronary syndrome. Ifechocardiography reveals right, rather than left heartdysfunction, immediate pulmonary angiography may be more appropriate than transferring anunstable patient to CT laboratory.
Non-High Risk Patients
In non-high risk patients the diagnostic strategyis focused not only on confirmation of PE (or DVTas both conditions result in the same therapeuticdecisions in hemodynamically stable patients). Evenmore importantly, diagnostic evaluation shouldidentify patients who, despite clinical suspicionof PE can be left without anticoagulation with anacceptably low risk of suffering any VTE episode inthe near future. This risk is defined in terms of thefrequency of any clinically evident VTE episode inthe subsequent 3 months, which should not exceed1%-2% (with upper limit of 95% CI of 3%) i.e. theexpected VTE rate after a negative pulmonaryangiography.26
Such "probabilistic" approach is necessary todeal with large numbers of patients who presentwith dyspnea, chest pain and/or other non-specificsymptoms and signs. Indeed only 25%-30% ofsuch patients will have PE confirmed by complete diagnostic evaluation. Therefore, the diagnosticevaluation should start with identification ofpatients with low to moderate pre-test clinicalprobability of PE according to Wells or Genevaprediction rules or according to subjectiveassessment.27,28 Such patients may be releasedwithout anticoagulation based on negativeplasma D-dimer result alone. Moderate sensitivityD-dimer tests are sufficient to make such adecision in patients with low clinical probability,while high sensitivity tests allow it also inpatients with intermediate clinical probability.29,30 Because D-dimer levels increase with age, comorbidities or pregnancy,30-33 the test is more useful for evaluation of previously healthy acutelyill patients of the emergency department.31,33-35 Patients with elevated D-dimer as well as thosewith high clinical probability require imagingtests, preferably multidetector CT angiography.36 Two landmark accuracy trails assessed diagnosticvalue of key non-invasive imaging methods inthe context of clinical probability of acute PE.PIOPED focused on lung scintigraphy37 and PIOPED II on multidetector CT (MDCT).38 Bothtrials revealed important influence of the pre-testprobability on the diagnostic performance of an individual test. Therefore, discrepancies betweenclinical and laboratory assessment needs furtherdiagnostic considerations. A negative MDCTresult in a patient with high clinical probabilityof PE, as well as a positive MDCT (limited tosubsegmental arteries) in a patient with low pre-test probability should be verified by additionaltests.39 Further increasing the number of CTdetectors may result in excessive reporting ofdistal, subsegmental pulmonary emboli, withunclear clinical significance.40
A concise list of diagnostic tests, and theirvalidated combinations accounting for clinicalprobability of PE, is given in Table 2.1 It mayoffer advice regarding construction of alternativediagnostic algorithms which must be sometimestailored to the limited local availability orlaboratory tests.
Diagnostic approach compatible with currentguidelines is related to better outcome41 and should be actively implemented. Modern hardware andsoftware may help in standardization of managementof patients with suspected and confirmed acutePE.42
Except for patients with low clinical probabilityof PE, and those with hemoptysis or other significant contraindications, anticoagulationwith low molecular weight heparin (LMWH)should be started upon clinical suspicion in orderto reduce the risk of early PE recurrence duringthe time needed to complete the diagnosticassessment.1
Recent attempts to simplify two previouslyvalidated prediction rules should be noted.43-46 In the Wells score a binominal scale ("unlikely-likely") has been suggested, instead of threelevels of pre-test clinical probability ("low-intermediate-high"). In addition, equal rank wasrecently assigned to all prediction score elements,apparently without significantly affecting itsperformance.43-45 Similar changes are being madein the Geneva score.46 Though currently someconfusion has been introduced, ultimately theseefforts may result in easier and wider use ofprediction rules.
New diagnostic methods and ideas are constantlybeing suggested. Bedside assessment of end-tidalCO2 has been proposed as a potential alternativeto D-dimer testing as an adjunct to Wells predictionrule.47 PIOPED II assessed the value of extendingCT angiography also to CT venography. However,the additional diagnostic yield was negligible anddid not justify increased exposure to radiation.38 Lung scintigraphy has been suggested as moreappropriate than CT in pregnancy, becauseof similar risk for the foetus but lower risk ofinducing maternal breast cancer.48,49 Potential role of new radiation-free tests such as thoracic50-52 and endobronchial ultrasound (EBUS)53 still needprospective validation trials to assess their value insuspected PE.
The role of magnetic resonance imaging (MRI) asa diagnostic test in PE has been suggested by smallertrials54-57 and has been recently defined in a large prospective PIOPED III trial. The main limitationof MRI was related to high rate of technicallyinadequate images, which ranged from 11% to52% (mean, 25%) in the 7 participating centers.Consequently, magnetic resonance angiographyidentified only 57% (59 of 104) of patients withobjectively confirmed PE. Technically adequatemagnetic resonance angiography had a sensitivity of78% and a specificity of 99% and when combinedwith magnetic resonance venography, 92% and 96%,respectively. Unfortunately only half of the patients(194 of 370) had technically adequate results of bothtests.58
Initial Treatment and ComprehensivePrognostic Assessment
Main recommendations for initial management ofacute PE are summarized in Table 3.
High Risk Pulmonary Thromboembolism
As already mentioned patients with acute PEat high-risk of early death are identified by thepresence of shock or systemic hypotension. Thetreatment goal in those patients consists not onlyof preventing early life-threatening recurrenceof emboli (initially with IV heparin) but aimsat prompt unloading of the RV. The latter canbe attempted and in most cases achieved byintravenous thrombolysis.59
Unfractionated heparin started immediatelyas a weight adjusted IV bolus (80 U/kg) followedby 18 U/kg/h and further activated prothrombintime (APTT)-adjusted infusion60 is preferred toLMWH in high-risk patients with PE. Thereis no consensus whether heparin should bediscontinued during the administration ofthrombolytic agent, and if so, when it should berestarted.
Out of three thrombolytic regimens formallyapproved for PE only alteplase (recombinant tissueplasminogen activator), 100 mg infusion over 2hours, with the first 10 mg usually given as bolusinjection, is currently used. Alternatively, fastinfusion of alteplase at the dosage of 0.6 mg/kg(maximum 50 mg) within 15 minutes can be used inemergency situations, eg, during cardiopulmonaryresuscitation.61 Bolus followed by prolongedstreptokinase or urokinase i.v. regimens have beenreplaced in clinical practice by more rapid, 1-2-hourhigh dose infusions. Those regimens were similarto those used in acute myocardial infarction, asthey achieve more rapid clot lysis at lower bleedingrisk.62
Satisfactory haemodynamic results also havebeen obtained in acute PE with double-bolusreteplase, 2 injections (10 U) 30 minutes apart63 or bolus tenecteplase.64 However, neither reteplase nortenecteplase are formally approved for treatment ofPE at present.
In patients with contraindications tothrombolysis and in approximately 10 % whofail to improve despite such therapy, surgicalembolectomy should be considered.65 It is worthreminding, that a patient with life-threateningPE is stabilized immediately after introducingcardio-pulmonary bypass. More recent seriesprovide reassuring data on the results ofsurgical embolectomy.66 Alternatively, catheterembolectomy, thrombus fragmentation, or both,may be considered, if adequate experience andequipment is available.67,68 If neither surgical norcatheter intervention are immediately available ina patient critically ill due to objectively confirmedPE hardly any contraindications preclude the useof thrombolysis.1 With the exception of recent cerebral or severe uncontrolled internal bleeding,thrombolysis should be considered, eg, inpatients with recent surgery. Severe hemorrhagiccomplications should be expected and promptlyhandled. Intrapulmonary administration ofthrombolytic agents is neither safer nor moreeffective than its systemic administration.68,69
Non-High Risk Pulmonary Thromboembolism
The main treatment goal in normotensivepatients with PE is an immediate and effectiveprevention of recurrences of emboli and of localextension of intrapulmonary thrombi. Thisshould be attempted and is usually achievedby anticoagulation, which allows intrinsicthrombolysis and thrombus retraction to prevail.Clearing of the pulmonary bed and restoringnormal hemodynamics may take weeks tomonths, and may not be complete.70
Weight-adjusted LMWH are the first choicetherapy for the majority of patients withdocumented acute PE,71-73 including thosepresenting with hemoptysis due to pulmonaryinfarction. Fondaparinux in three fixed dosesdepending on the body weight (5 mg for patientswith <50 kg, 7.5 mg for patients with 50-100 kg and10 mg for patients with >100 kg of body weight) is a valid alternative,74 particularly in patientswith renal insufficiency as it allows non-modifiedadministration down to glomerular filtration rate(GFR) of 20 mL/kg/min, compared to 30 mL/kg/min for the LMWH. Fondaparinux is probablynot inducing PF4 antiplatelet antibodies and "heparin induced" thrombocytopenia. In contrastto LMWH it should not be used in pregnancy dueto lack of evidence. LMWH treatment does notrequire laboratory monitoring, except in extremesof body weight, significantly reduced renalclearance and pre-delivery period in pregnancy. Insuch situations anti Xa activity-guided treatmentmay be considered.71 Tinzaparine, enoxaparineand—for cancer patients—dalteparine have formallabeling for PE. However, it is common practiceto extrapolate existing evidence to other LMWHs,which have documented efficacy in treatment ofDVT.
Unfractionated i.v. heparin is preferred to LMWHin several clinical situations, including: unstable and "high-risk" PE, significant bleeding risk, severe renalfailure. Adequately high dose of UFH is crucialfor successful prevention of recurrent PE episodes.Daily IV dose ≥40 000 U should be effective even incases without adequate APTT prolongation (definedas >1.5 to 2.5 control value), though monitoring ofanti-Xa would be even more reassuring.75
Anticoagulation initiated with heparins orFondaparinux should be continued with avitamin K antagonist (VKA). Oral VKA maybe started already on the first day of therapyand continued in parallel with a parenteralanticoagulant in therapeutic doses for at least4 days. The latter can be stopped only afterbringing the international nomalizing ratio (INR)to the target range, ie, 2.0-3.0 for ≥ 2 consecutivedays.71 In selected patients in whom optimal INRmonitoring seems difficult, LMWH may be usedfor secondary prevention at doses recommendedby the manufacturer for such purpose, usuallyrepresenting 50%-75% of the full therapeuticdose.1 Pregnancy represent specific situation inwhich most experts suggest LMWH dose at 75%-100% of therapeutic dose until delivery, becauseof its increased clearance.76
Thrombofilia does not require modification ofinitial therapy, with the exception of significantantithrombin deficiency. It may result in resistance tounfractionated heparin manifesting as lack of APTTprolongation and can be corrected by increasing thedose of UFH or by substitution of antithrombin.The effect of antithrombin deficiency on LMWHefficacy is less clear.
Intermediate Risk Pulmonary Thromboembolism
Thrombolysis may be also considered in selectedpatients, who do not meet the criteria for high risk ofearly PE-related death.1 Comprehensive prognosticevaluation by searching for RV pressure overload/dysfunction and/or myocardial injury may identifynormotensive patients who are at relatively higherrisk
Echocardiography was considered a key testpredicting in-hospital outcome in acute PE.25,77-80 This has been questioned by a recent meta-analysisincluding 475 normotensive patients with PE whichreported only moderate negative (60%) and positive(58%) value of echocardiography for predicting earlydeath.81 Standardization of the echocardiographiccriteria which could be universally applied forprognostic staging in acute PE remains an unresolvedissue.82
MDCT, currently the preferred method fordiagnosing PE, may simultaneously detect RVenlargement due to PE and such finding hasprognostic implications.83 A meta-analysis of 2studies including 191 normotensive patients with PEreported a 58% overall negative and a 57% positivevalue of RV dilatation on CT for predicting earlydeath.81
Natriuretic peptides offer a non-imaging insightinto ventricular dysfunction, including that caused by acute PE.84-87 A meta-analysis including 1,132patients found increased plasma BNP/NT-proBNPlevels to be related to significant risk of early death(OR, 7.6; 95% CI, 3.4-17).88 The prognostic valueof natriuretic peptides may be improved whenconsidered together with echocardiography89 and/orclinical data.90
While all the above markers of RV dysfunctionseem useful for prognostic stratification innormotensive, i.e. otherwise "non-high risk" patientswith PE, no universal cut-off values were defined andno therapeutic recommendations can be formulatedat present. Particularly RV overload/dysfunctionalone does not appear to justify rutine use of moreinvasive treatment regimens such as thrombolysis orembolectomy.24
Just as in acute coronary syndromes cardiactroponins can be detected in up to 50% of patientswith acute PE.91 A metaanalysis enrolling 1,985patients from 20 studies reported increased riskof death (OR, 5.24; 95% CI, 3.28-8.38) in patientswith elevated troponin levels.92 When a similarassessment was restricted to 1,366 normotensivepatients patients enrolled in 9 studies troponinelevation alone was not found to contributesatisfactorily to prognostic staging.93 The value of high sensitivity troponin tests in PE remainsto be evaluated. Other potentially prognosticallyuseful markers of myocardial injury or ischemia inPE include heart-type fatty acid-binding proteins(H-FABP)94-97 and growth-differentiation factor-15(GDF-15).98
Unfortunately, the positive predictive value formortality is low and the optimal cut-off point isnot universally established for any of the individualbiomarkers indicating myocardial injury. Even asingle risk marker found "positive" according tolocal criteria is sufficient to consider a patient asone at "intermediate-risk" of early death (3%-15%in hospital or 30 days mortality). Possible additivevalue of signs of myocardial injury and dysfunctionis likely. Since approximately 25% of intermediate-risk patients will have a complicated clinical course,24 they should be considered for close monitoring eitherby telemetry or at the intensive care unit , to allowearly "rescue" therapy (so called "watchful waiting" strategy). Results of a long awaited randomizedcontrolled study assessing potential benefit ofthrombolysis over heparin alone in patients withacute PE presenting with echocardiographic signsof RV overload an increased plasma troponin(PEITHO) should be available in 2012.
Low-Risk Pulmonary Thromboembolism
Low risk PE can be diagnosed in patients inwhom markers of RV dysfunction and myocardial damage were tested but found to be negative.However, outcome may additionally be influencedby comorbidities and general condition of thepatient. Recently, a Pulmonary Embolism SeverityIndex (PESI) was validated in large populationsof patients with PE99,100 and found capable of identifying patients with very low rates of adverseevents.101 The index considers such factors likeage, sex, comorbidities, presence of tachycardia,tachypnea, hypotension, hypothermia, hypoxemiaand confusion. Low PESI index may help indecisions regarding early discharge and hometreatment of low-risk patients with acute PE.
Long-Term Secondary Prevention
Prevention of recurrence is a priority aftera documented PE episode. Without it up to50% of patients may suffer a recurrent episodewithin the first 3 months.102 While provoked PErequires only 3 months of anticoagulation withnegligible risk of late recurrence, unprovoked PEis considered a lifelong disease. The frequency ofrecurrence appears to be independent of the initialclinical manifestation of VTE, but recurrent VTEis three times more likely to present as PE if theinitial clinical event was PE, than if it was DVT.103 However, the majority of available data refer toDVT rather than PE alone and indicate at least 30%recurrence rate after 8-10 years.104-106 Treatment with VKA is highly effective in reducing the riskfor recurrent thromboembolism by up to 90%.107
However, the risk of recurrence returns after theirdiscontinuation, regardless of the duration oftherapy.108,109 After unprovoked PE indications forlonger or indefinite oral anticoagulation should beassessed on an individual basis after at least 3 monthsof initial secondary prevention. This population ofpatients is in clear need of additional markers forfurther risk stratification for VTE recurrence. Somehelp is offered by D-dimer testing one month afterdiscontinuation of vitamin K antagonists. Patientswith abnormal D-dimer plasma levels shouldresume anticoagulation, because of relatively highrisk of recurrent events.110 Persistent thromboticdeposits detected by CUS in deep venous systemrepresent another marker of increased recurrencerisk in idiopathic PE. 111
While effective, routine prescription of indefiniteanticoagulant prevention is questionable inview of the resulting increased risk of majorbleeding.1,71,107,112 In fact, chronic anticoagulation prevents recurrent VTE at a cost of major bleedingrate of 3-4% within clinical trials, and up to 5%-9% in everyday clinical practice.113 Bleeding complications during the first 3 months of therapyare strong determinants of mortality. Out of 407 patients followed in RIETE registry who hadmajor bleeding during the study period 133 (33%)died in the next 30 days -half of them because ofbleeding-.114 Periodical reassessment of indicationsand contraindications to continued VTE prevention,accounting also for patient's preferences, istherefore important.71 Double antiplatelet therapiesfollowing many cardiovascular interventionsrepresent a new challenge for prophylactic longterm anticoagulation. Venous filters seem toreduce mortality when inserted because of bleedingcomplications in patients receiving anticoagulantsup to 3 months after a VTE episode. In the RIETEregistry insertion of a vena cava filter was the onlyvariable independently associated with a lowerincidence of fatal bleeding (OR, 0.10; 95% CI, 0.01-0.79) and all-cause mortality (OR, 0.21; 95% CI, 0.07-0.63). Stopping anticoagulation was related toincreased risk of death (OR, 2.31; 95% CI, 1.37-3.94).114
Indefinite anticoagulation is recommendedafter a second unprovoked episode of VTE.Patients with thrombophilia or active cancer arealso candidates for indefinite anticoagulationwith VKA. Patients with cancer require secondaryprevention with LMWH instead of VKA, as itseems to improve their outcome at least whengiven during the first 6 months after an acuteVTE event.115,116
New generation oral anticoagulants, mostly antiXa, and direct antithrombin agents are currentlyunder investigation for prophylaxis and treatmentof VTE and may help in improving the balancebetween efficacy of prevention and the risk ofbleeding.117
CHRONIC THROMBOEMBOLIC DISEASE
Chronic thromboembolic pulmonaryhypertension (CTEPH) can be diagnosed iforganized thrombi in main, lobar, segmentalor subsegmental pulmonary arteries can bevisualized in a patient with precapillary pulmonaryhypertension ie, with mean pulmonary arterypressure (PAP) ≥25 mmHg, pulmonary vascularresistance (PVR) >2 IU and pulmonary arteryocclusion pressure ≤15 mmHg.
Epidemiology and Predisposing FactorsCTEPH is a rare consequence of acute PE. Untilrecently it was believed, that only 0.1%-0.5% ofpatients with acute PE develop CETPH,118 while vast majority clear their pulmonary bed fromthromboemboli mostly by means of endogeneousthrombolytic activity.119 More recent reports suggest higher prevalence of CTEPH reaching 3.1% and 3.8%, at one and 2 years after an embolicepisode, respectively.120 A multicentre prospectiveobservation of 259 patients after a first episodeof PE revealed overall 0.8% incidence of CTEPHduring 46 months of observation, with twice as high (1.5%) incidence among patients with idiopathicPE.121
CTEPH is considered to be primarily dueto aborted endogenous thrombolysis after athromboembolic episode.122 Few pro-thromboticrisk factors have been identified in subjects with thedisease and only 50% of patients with documentedCTEPH have traceable history of acute PE.123 Other medical conditions associated with CTEPHinclude splenectomy, ventricular valve for treatmentof hydrocephalus, chronic osteomyelitis andinflammatory bowel disease.124
Diagnostic Approach
Any new limitation in exercise capacitydue to dyspnea, requires consideration ofCTEPH among its potential causes. This is trueparticularly—but not only—in patients witha history of venous thromboembolic disease.CTEPH should be also considered in all patientswith echocardiographic signs suggesting RVpressure overload, in whom common causes ofPH have been excluded.
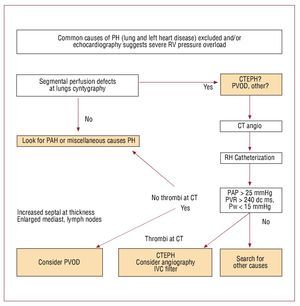
Prospective echocardiographic screening ofasymptomatic survivors of acute PE for CTEPHis questionable. A recent Dutch prospectivescreening study enrolling 866 patients withhistory of acute PE revealed 0.57% (95% CI, 0.02-1.2) prevalence of CTEPH , again higher (1.5%;95% CI, 0.08-3.1) in idiopathic cases. However,most of the patients with CTEPH were alreadyidentified because of clinical symptoms and signs.This happened before they were invited for formalechocardiographic screening, which had very lowadditional diagnostic yield for CTEPH, and wasnot found practically useful by the authors.125 An algorithm which may be useful for planningdiagnostic strategy in suspected CTEPH is shownin figure 2
Figure 2. Suggested diagnostic approach to patients with echocardiographic signs of unexplained right ventricular pressure overload. CT indicates computed tomography; CTEPH, chronic thromboembolic pulmonary hypertension; IVC, inferior vena cava; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PVOD, pulmonary venoocclusive disease; PVR, pulmonary vascular resistance; RH, right heart; RV, right ventricle; Pw, wedge pressure; PAH,pulmonary arterial hypertension.
Contrary to acute PE, lung perfusionscintigraphy maintained an important position inthe differential diagnosis of chronic pulmonaryhypertension. It is an excellent screening tool forCTEPH.126 Normal perfusion scan excludes CTEPHwhile multiple defects prompt further diagnosticimaging. CT angiography is a recommended nextstep. If mural thrombi, intraluminal bands orwebs can be visualized CTEPH is highly probable.Mozaic perfusion pattern on high resolution CTis a common finding supporting the diagnosis.Depending on the extension and character of intrapulmonary lesions, and local experienceclassical pulmonary angiography may or may notbe needed for surgical qualification. If performed,it helps to identify not only mural organizedpost-thrombotic deposits but also residual websand bands which represent fibrotic remnants ofthrombi and may contribute to increased PVR.Intravascular changes are therefore differentin CTEPH than those encountered in acutePE.126 Of note, organized thrombi in proximalpulmonary arteries may be found in pulmonaryarterial hypertension, particularly in patientswith Eisenmenger syndrome. Such deposits resultfrom local stasis in markedly dilated arteries, anddo not have direct hemodynamic consequences,except for potential artery-to-artery embolization.Anomalies of pulmonary arteries, vascular tumors(such as angiosarcoma, leyomyoma), Takayasuarteritis and mediastinal fibrosis may sometimescause major diagnostic problems, mimickingCTEPH when assessed with imaging methods.127-129
Interpretation of images as well as diagnosticand therapeutic decisions in suspected CTEPHrequires particular experience, multidisciplinaryapproach and therefore should be restricted tospecialized referral centers.
Treatment
Advanced CTEPH, if left untreated carriesa very poor prognosis. This is due not only topersistent post-embolic deposits but remodelingof pulmonary arterioles similar to those found inpulmonary arterial hypertension, progressivelyincreasing RV afterload.130 Historical datareferring to patients with CTEPH remainingon supportive therapy alone suggest 30% to80% mortality depending on their mean PAPat presentation (>30 mmHg and >50 mmHg,respectively).131 Patients with CTEPH but amean PAP <30 mmHg had 12% mortality during 18.7 months of follow-up which contrasted with50% mortality in those with mean PAP >30mmHg.132
Surgical Therapy
Surgical pulmonary endarteriectomy (PEA) isthe preferred mode of treatment of patients withCTEPH.123,126
The successful intervention was reported in1973 by Kenneth Moser and Nina Braunwaldfrom UC San Diego.133 Since that time PEA isperformed with the help of cardio-pulmonaryby-pass and requires remittent periods of deephypothermia. This prevents from back-bleedingfrom bronchial circulation and allows removal of pulmonary artery intima down to segmentaland sometimes sub-segmental branches, togetherwith attached intraluminal organized post-thrombotic deposits. If complete bilateralendarterectomy is technically successful andpotential perioperative complications, suchas reperfusion lung oedema or bleeding, arecontrolled clinical, hemodynamic and long-termprognostic improvement can be dramatic.134 Most centers routinely implant vena cava filters beforePEA to prevent peri-operative or late recurrenceof PE. The latter sometimes occur in patientswith excellent result of PEA, who may neglectchronic anticoagulation, considering themselvescured of the disease. Recently, some surgeonsperforming PEA advice against implantationof vena cava filters, as they may interfere withimplementation of extracorporeal life supportsystems (ECLS). Such interventions may be life-saving in patients with severe respiratory andRV failure developing in the early post-surgicalperiod.135 Retrievable venous filters might be asolution but in this clinical setting the experienceis still lacking.
Not in all patients endarterectomy can be effective.This depends mostly on the relative contribution ofproximal postthrombotic and distal proliferativeelement to the elevation of PVR and to increase inRV afterload. Jamieson and Kapelaski describedfour types of intrapulmonary findings revealedduring PEA interventions, and correlating with theiroutcome.136 In the majority of potential surgicalcandidates with CTEPH the decision regardingtheir operability is relatively clear. In others moresophisticated methods based on the Doppler-assessedsite of reflected PAP wave, assessing partitioning ofPVR or pulmonary vasoreactivity tests may offersome help.137-140 However, there is still a significant group of patients in whom there is no method whichwould allow unequivocal preoperative prediction ofthe final hemodynamic result of surgery.
Universally accepted indications for PEAinclude:
- III or IV WHO functional class.
- PVR >300 dyn¿s¿cm-5.
- Proximal changes localized in main, lobar,segmental pulmonary arteries.
- Absence of severe comorbidities.
Main indicators for successful outcome of PEAinclude141:
- Surgeon and team experience.
- Concordance between PVR and % occlusion ofpulmonary artery bed.
- Preoperative PVR <1000-1200 dyn¿s¿cm-5.
- Absence of specific comorbidities (splenectomy,ventriculo-atrial shunt).
- Early postoperative PVR < 500 dyn¿s¿cm-5.
In some patients complete unilateral occlusionresult in exercise limitation despite relatively mildhemodynamic disturbances at rest. This is duemostly to increased dead space in the non-perfusedlung and may also represent an indication forPEA.142 However, for not completely clear reasonsunilateral pulmonary artery occlusions are relatedto high risk of recurrences after PEA,143 and this should be considered when qualifying a patient forsurgery.
Experience and multidisciplinary approach isessential in achieving success in CTEPH surgery.Data from the center in San Diego, where over 2000PEA was performed the mortality from initial 20%was reduced to 4.5% for the interventions performedafter 2004.144,145
Medical Therapy
All CTEPH patients should receive life-longanticoagulation, usually with a VKA, to prevent boththe recurrence of PE and local extension of thrombiin pulmonary arteries, arterioles and pulmonarymicrocirculation.124 It is true both before and after PEA (including patients with implanted vena cavafilters) as well as in patients who remain on medicaltreatment alone
In symptomatic patients supportive measuressimilar to those recommended in PAH should beconsidered.2 These include diuretics in patientswith RV failure and oxygen supplementation inhypoxemic patients. Vaccinations against influenza,avoiding pregnancy and excessive physical activityshould be also suggested.
Despite improvement in surgical techniqueand experience still almost 50% of patients withsymptomatic CTEPH remain on medical treatmentalone. This is mostly because of inoperable distalchanges or comorbidities.123,141,145 In addition in about 10% of patients pulmonary hypertensionpersists after PEA, because of the remainingdistal deposits or the contribution of arteriolarremodeling.123,141,145 With increasingly encouragingevidence on vasodilative and anti-remodeling therapy in pulmonary arterial hypertension146 potential indications for targeted medicaltreatment in CTEPH emerged and stimulatedclinical trials.
Potential indications for medical therapy inCTEPH include:
- Distal disease considered inoperable.
- Comorbidities prohibitively increasing risk forsurgery.
- Bridge to PEA or transplantation for high-riskpatients.
- Persistent pulmonary hypertension despitePEA.
Several small pilot series and case controltrials supported the concept of targeted medicaltherapy in CTEPH. However, reliable evidencefrom prospective randomized controlled trialsincluding a placebo group is still inconclusive.The main results from three trials which havebeen undertaken in CTEPH147-149 are listed in Table 4. Two of those trials were small. The onlyone powered to detect a statistical differencebetween the active treatment and control was theBENEFIT trial. It included patients with eitherinoperable CTEPH or pulmonary hypertensionpersisting >6 months after PEA. Independentco-primary end points were change in PVR as apercentage of baseline and change from baselinein 6-min walk distance after 16 weeks of treatmentwith bosentan 125 bid or placebo. Secondaryend points included change from baseline inthe World Heart Organization functional classand other hemodynamic parameters. The trialshowed a statistically significant treatment effectof bosentan over placebo on PVR with its 24.1%reduction from baseline (95% CI, -31.5 to -16.0; P<.0001) after 16 weeks.147 The other co-primaryendpoint, distance walked during 6 minute walktest was not met, with mean improvement inactive versus placebo group of only +2.2 m (95%CI, -22.5 to 26.8 m; P=.5449). Several clinicallyrelevant parameters significantly improved inpatients randomised to bosentan when comparedto those on placebo, including total pulmonaryresistance and cardiac index as well as NT-proBNP plasma levels. Bosentan treatmentwas well tolerated. How to explain discrepancybetween hemodynamic and functional resultsremains unclear. Physical deconditioning delayingfunctional recovery in the CTEPH patients, dueto comorbidities and older age, as compared toPAH, has been suggested.
In a recent retrospective observational study355 patients treated with PEA at San Diegobetween 2005-2007 apparently did not benefit from preoperative treatment with bosentan,sildenafil or epoprostenol (as a monotherapy orin combinations) when compared to 244 patientswho received supportive treatment alone.Targeted therapy resulted in delayed surgicalreferrals, but did not influence the postoperativecourse.150 Despite those inconclusive data asignificant proportion of CTEPH patientsreceive targeted pharmacotherapy worldwide.Recently completed large European CTEPH
Registry should provide further informationon the current diagnostic and therapeuticstrategies and outcome of patients with CTEPHin Europe. Even more importantly most of thenew drugs tested for treatment of pulmonaryarterial hypertension are also in parallel testedin inoperable and "persistent" CTEPH patients.This should help in objective assessment of thevalue and place of specific medical therapy forthese indications.
ABBREVIATIONS
CTEPH: chronic thromboembolic pulmonaryhypertension
DVT: deep vein thrombosis
LMWH: low-molecular-weight heparin
MDCT: multidetector computed tomography
PE: pulmonary thromboembolism
RV: right ventricle
UFH: unfractionated heparin
Disclosure: Adam Torbicki reports serving as a consultant for Actelion,Bayer Schering, Eli Lilly, GlaxoSmithKline, and mondoBIOTECH; receivedlecturing honoraria from Bayer Schering, Eli Lilly, Actelion, Pfizer andSanofi Aventis; conducted research supported by Actelion, BayerSchering, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Sanofi Aventis,mondoBIOTECH, and Pfizer.
Correspondence: Prof. A. Torbicki.
Department of Chest Medicine. Institute of Tuberculosis and LungDiseases.
ul Plocka 26, 01-138 Warsaw. Poland.
E-mail: a.torbicki@igichp.edu.pl