Keywords
INTRODUCTION
Mitral regurgitation (MR) is characterized by flow reversal in systole from the left ventricle (LV) into the left atrium. The etiology of MR is predominantly primary or organic (intrinsic alteration of the leaflets), and in developed countries is most commonly degenerative. In less developed countries, the etiology is most frequently rheumatic. Functional MR (normal leaflets with annular dilation or apical displacement of the point of apposition of the leaflets -tenting- secondary to dilated cardiomyopathy or LV ischemia) continues to increase due to the global epidemic of heart disease. Chronic organic MR is the most common valvular disease in the United States, with a prevalence of moderate or severe MR of 1.7% in the general population, 6.4% of individuals between 65 and 74 years, and 9.3% in those aged >75 years.1 Doppler echocardiography has proved to be the ultimate non-invasive method for diagnosis and treatment decision-making. This review aims to discuss current thinking on the natural history, progression, consequences, and treatment of severe MR. We propose an algorithm that emphasizes the importance of a comprehensive echocardiographic assessment of MR as a starting point, followed by consideration of multiple clinical factors which should be applied on an individual basis to obtain the best treatment plan for each patient.
TREATMENT OF ACUTE MITRAL REGURGITATION
Acute IM constitutes a surgical emergency. It has a mortality rate of 15%-20% at 30 days.2 Common causes are chordal rupture, bacterial endocarditis, ischemic rupture of the papillary muscle, acute rheumatic carditis, acute cardiomyopathy, and acute prosthetic dysfunction. The affected patient presents dyspnea, hemodynamic instability, and symptoms of shock. However, some patients may have dyspnea without clinical hemodynamic deterioration. These patients may be misdiagnosed with another process, because physical examination may not provide clear evidence of a slight systolic murmur. This makes the physician's clinical suspicion essential. The transthoracic echocardiogram shows a normalsized hyperdynamic LV and an MR jet that may be inconspicuous, due to the predominance of the jet's potential energy (giant V-wave pressure) as opposed to its kinetic energy (speed). The transesophageal echocardiogram in this case is crucial, because it reveals the mechanism underlying the MR and provides a clearer picture of its severity. Medical therapy with vasodilators, vasopressors, and aortic balloon are used to stabilize the patient in preparation for immediate surgery, but should not delay surgery. Up to 7% of patients with ischemic cardiogenic shock (acute) have severe MR, and mortality is higher than in those without MR. Mortality is reduced with immediate revascularization so that, where surgical revascularization is indicated, the presence of severe MR should be a motive for even quicker implementation.2
NATURAL HISTORY OF SEVERE CHRONIC MITRAL REGURGITATION
Mitral regurgitation is a progressive disease, with average annual increases of 5-8 mL in regurgitant volume (RVol) and 4-6 mm2 in the effective regurgitant orifice (ERO). Anatomical changes are determinants of progression, which is faster in patients with mitral valve prolapse, especially in those with ruptured chordae (up to 20 mL per year), and in patients with mitral annular dilatation. The progression of MR can lead to adverse LV remodeling, and development of ventricular dysfunction.3 In these cases, ejection fraction (EF) may initially be "normal" (>50%) and symptoms may not be present, though prognosis is worse than in the unaffected population. Published estimates on long-term survival in patients with MR vary considerably.4,5 At the Mayo Clinic, we analyzed the natural history of MR caused by ruptured chordae tendineae6 and found significant mortality in MR (6.3% annually) compared with expected survival in the general population. There was also substantial morbidity at 10 years, with incidences of atrial fibrillation (AF) and heart failure of 30% and 63%, respectively. At 10 years, 90% of patients had died or undergone surgery, making the operation almost inevitable in this population. Patients who were even temporarily in New York Heart Association (NYHA) functional class III-IV showed high mortality (34% annually) if they were not operated on, but mortality was high (4.1% annually) even among those in functional class I-II. Patients with EF <60% also showed excess mortality compared to those with EF >60% on medical treatment. So waiting for a reduction in EF to <60%, or for the appearance of NYHA functional class II symptoms does not seem appropriate, as prognosis with treatment will be worse.
Sudden death is a cataclysmic event in severe MR with rupture of chordae tendineae and causes about 25% of deaths of patients on medical treatment.7 Overall, the sudden death rate is 1.8% annually and even in patients without other risk factors (severe symptoms, LV dysfunction, and HF) is 0.8% per year. These data highlight the poor prognosis implied by severe organic MR, especially when ruptured chordae are involved. This is likely due to their being associated with massive MR (RVol >100 mL per beat and ERO >0.5 cm2) in 85% of cases.
LEFT VENTRICULAR DYSFUNCTION: ECHOCARDIOGRAPHIC PARAMETERS
During ventricular systole, the RVol of MR heads to the left atrium with decreased ejection impedance, and afterload remains normal. A normal afterload coupled with volume overload can maintain a normal or supranormal EF for a considerable time, and irreversible LV dysfunction can coexist with normal EF.
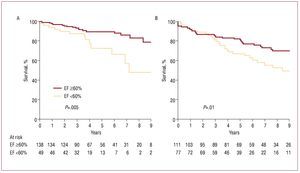
The EF and end-systolic diameter (ESD) of the LV are the most practical parameters for evaluating the LV as they are easy to quantify using an echocardiograph and because of their predictive power (they represent an approximation to intrinsic LV contractility). Left ventricular dysfunction (EF <60%) indicates poor prognosis with conservative treatment6 and even postoperatively8 regardless of the type of surgical correction used (Figure 1). Although advances in anesthesia and myocardial protection have meant that perioperative death is rare, LV dysfunction is still the most common cause of late postoperative death.8 Thus, despite an improvement in symptoms, LV dysfunction occurs in about one third of patients with organic MR who are operated on successfully. Such dysfunction is associated with poor survival3,9 and a high incidence of heart failure,10 which must be prevented.
Figure 1. Kaplan-Meier curves showing postoperative survival in severe mitral regurgitation, based on ejection fraction and surgical technique. A: although mitral repair is carried out, postoperative survival decreases if preoperative ejection fraction is <60%. B: the same result is obtained with mitral replacement. Reprinted from Enriquez-Sarano et al,8 with permission from the American Heart Association. EF indicates ejection fraction.
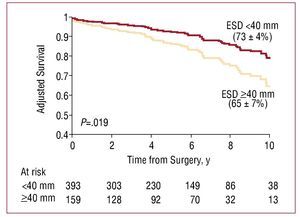
The ESD is also essential in predicting the reduction in postoperative EF3 and survival. It has recently been shown that waiting for the ESD to reach ≥40 mm is independently associated with poorer postoperative survival in the medium and long term in severe MR (Figure 2).11
Figure 2. Kaplan-Meier postoperative survival curves in severe mitral regurgitation by end systolic diameter (ESD). Survival is significantly reduced in patients with ESD ≥40 mm, independently of whether symptoms are present. Reproduced from Tribouilloy et al,11 with permission from Elsevier.
Subjects with EF <60% and / or ESD ≥40 mm should be considered as patients with manifest LV dysfunction even if they are asymptomatic, and, where it is not contraindicated, they should receive salvage surgery12 (Table 1). This implies a discrepancy between current recommendations (Table 1) and those of the current authors (see below), but using EF <60% and ESD ≥40 mm as indicators for surgery may involve too long a delay and will lead to poorer medium and long term survival (Figures 1 and 2).
MITRAL SURGERY: RECENT ADVANCES
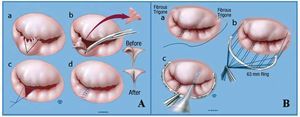
Mitral regurgitation is the result of a mechanical dysfunction of the mitral valve and definitive correction is also mechanical (surgical). The mitral valve is not an isolated entity; it is closely related to the LV and ensures the latter's geometry and function via the connection through the chordae tendineae. For that reason, mitral repair with preservation of the subvalvular apparatus forms the basis of current surgical treatment. In patients with organic MR, operative mortality has decreased considerably,13 especially with mitral repair (Figure 3). In the Mayo Clinic in Rochester, operative mortality is around 1% in patients under 75 years in cases of mitral valve repair or replacement,8 and <1% in isolated repair. By contrast, operative mortality in patients over 75 years is approximately 5% in our institution. However, surgery in patients over 75 years leads to survival which approximates life expectancy in these patients to that of the general population.14 Surgery in organic disease (usually fibroelastic deficiency in this age group) should therefore be considered in these patients to prevent LV dysfunction and restore normal survival.
Figure 3. Mitral repair. Basic surgical concepts. A: a, P2 segment of the posterior leaflet without support due to chord rupture; outline of triangular resection (dotted line); b, triangular resection of P2; c and d, apposition and suturing of free edges. B: suturing of a 63 mm band (standard at the Mayo Clinic) to the fibrous trigones of the mitral ring (a and b), followed by integrity test with saline solution (c).
Previous studies indicate lower perioperative mortality and greater long-term survival after valve repair.15,16 In our experience, after adjusting for clinical differences among patients receiving repair versus replacement, valve repair per se is an independent predictor of better outcomes after surgery for MR.10 The experience of the surgical center is essential to achieving optimal results. Hospitals performing over 140 repairs per year achieve surgical mortality rates of close to 1%, compared to 3% in hospitals performing fewer than 140 repairs per year.17 Intraoperative transesophageal echocardiography is an essential component in successful valve repair and should be performed by experienced clinicians. They should supervise the procedure and assist in intraoperative decision-making.18
Currently, success rates in mitral repair are around 90%-95%. This high rate of success is possible once the surgeon's initial learning curve is completed and by using special techniques such as chordal transposition or insertion of artificial chordae, in particular to repair ruptured chordae tendineae in the anterior leaflet.19,20
Unfortunately, mitral repair in rheumatic lesions is not as successful.21 However, mitral repair in rheumatic MR should be performed when anatomically and functionally possible, because it is associated with longer survival than valve replacement. Mitral replacement decreases the need for re-intervention in rheumatic MR, but limits survival and increases the risk of embolic complications.22
TIMING OF SURGERY
There are 2 strands in current thinking as to when surgery should be performed in severe MR. One of these recommends "watchful waiting" until the onset of symptoms or indicators of subclinical dysfunction in asymptomatic patients (eg, EF <60%, ESD LV>40 mm), with echocardiographic evaluation of the patient every 6 or 12 months. The other approach recommends considering "early mitral repair," ie, intervening before symptoms or indicators of subclinical LV dysfunction are present. This approach stresses the need for sufficient evidence of MR severity, obtained by appropriate echocardiographic quantification (with information on the values of RVol and ERO), and a likelihood of successful repair of >95%, with operative mortality of 1% or less. These conditions require a cardiologist who is able to quantify the MR and critically evaluate the patient, a surgeon with experience in mitral repair, and an experienced postoperative team. Estimation of MR severity should include quantitative methods and indicators or existing support (Table 2).23 The most widely used quantitative techniques are Doppler quantification and the PISA (proximal isovelocity surface area) method. Both are based on the analysis of proximal flow convergence in the ERO region24,25 and allow for the calculation of RVol and ERO.26,27
However, we believe that the two strands of thought mentioned are not mutually exclusive but are complementary, and indicate the spectrum of severity and risk in severe MR. In other words, patients with severe MR are not all the same. In the Mayo Clinic, 198 asymptomatic patients with severe MR (mean age, 63 years; mean RVol, 66 mL/beat; mean ERO, 0.4 cm2) were studied prospectively. Mean LV and diastolic ESDs were 37 mm and 61 mm, respectively.28 We found that an ERO >0.4 cm2 in asymptomatic patients was independently associated with decreased survival at 5 years (58% compared to 78%, P=.03) while surgical repair was associated with better outcome. We therefore recommend consideration of early surgery in asymptomatic patients with severe MR. The incidence of clinical events in these patients was 10%-15% per year. By contrast, Rosenhek et al29 prospectively evaluated 132 asymptomatic patients with severe organic MR, and found that survival at 8 years was similar to that in the general population when "watchful waiting" to the onset of symptoms or echocardiographic indicators was employed. They concluded that this strategy was therefore appropriate. However, the average age of these patients was 55 years, LV and diastolic ESD were 34 mm and 56 mm (severe MR), and MR was not quantified using quantitative Doppler or PISA techniques. The incidence of total medical events was also much lower than in the previous study. The latter study therefore treated younger patients with likely moderate-severe MR, while ours included patients with more severe MR. A third group of researchers, Kang et al30 studied 447 consecutive patients with severe organic MR (mean age, 50 years; mean ERO, 0.8 cm2) managed using "early mitral repair" or "watchful waiting." A propensity matching subgroup of 127 pairs of patients was used to control for basic clinical features, and a high percentage (44%-46%) had ruptured chordae tendinaea; mean LV and diastolic ESD were 36 mm and 59 mm, respectively. Early elective surgical repair was associated with no mortality at 7 years, compared to 5% at 7 years for "watchful waiting." This third study was similar to ours in terms of echocardiographic severity and degree of LV dilation, although patients were younger. Thus, none of the studies were wrong, they just included different patients with different degrees of severity within an overall severe MR.
NOT ALL SEVERE ORGANIC MITRAL REGURGITATIONS ARE EQUAL: RISK FACTORS
From the previous discussion it follows that there are different levels and risk factors in severe MR (Table 3). The first risk factor is age. Studies agree that patients >55 years are at greater risk of complications, probably because of decreased contractile reserve associated with age. However, it is difficult to establish a cut-off age above which risk increases significantly. Chordal rupture and / or massive MR (RVol >100 mL and ERO >0.5 cm2) are risk factors within the spectrum of severe MR. The annual incidence of AF is 5% in patients with significant organic MR, and its appearance is associated with decreased survival.31 Even after valve repair, the presence of AF prior to the repair is associated with decreased survival at 5 years compared to normal sinus rhythm before repair (87% compared to 96%,P=.002).32 Increased levels of brain natriuretic peptide (BNP) represent another risk factor, which was assessed in a prospective study of 124 patients with organic MR. Raised BNP levels are related to the consequences of regurgitation (eg, LV and left atrium volume, AF, and symptoms) and is an independent predictor of mortality and heart failure, regardless of whether symptoms are present or not.33 This concept has been reassessed and validated prospectively in 269 consecutive patients, and it has been shown that a BNP value of ≥105 pg/ mL is associated with 4.6 times greater risk of heart failure, decreased EF and death in asymptomatic patients with severe organic MR.34
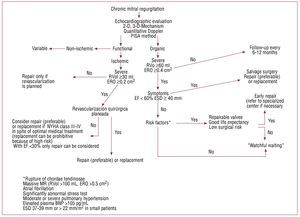
The presence of pulmonary hypertension observed using echocardiography is another risk factor predicting a worse prognosis.12 Recent analysis using data from the MIDA registry showed that risk of death is 2.5 times higher in patients with severe organic MR and pulmonary hypertension (unpublished data). Finally, whether or not the patient recognizes symptoms may be influenced by idiosyncratic differences in perception, fear of surgery, or because he / she performs little physical activity or has adapted levels of physical activity to accommodate the limitations imposed by mild symptoms. In 134 patients with severe organic MR and normal EF who self-classified as asymptomatic, we found that 1 in every 4-5 had a significant reduction in peak oxygen consumption (<85% of predicted values by age and sex) with evidence of limitation in exercise cardiac output and low functional capacity. These "asymptomatic" patients turned out to have greater number of adverse events or surgery within 3 years of follow-up.35 It is therefore essential to stress test patients when there is any degree of uncertainty about symptoms. These risk factors should be taken into account when deciding the appropriate time for surgery for each patient (Figure 4).
Figure 4. Algorithm for treatment of mitral regurgitation. BNP indiates brain natriuretic peptide; ESD, end systolic diameter; EF, ejection fraction; ERO, effective regurgitant orifice; MR, mitral regurgitation, NYHA, New York Heart Association; RVol, regurgitant volume.
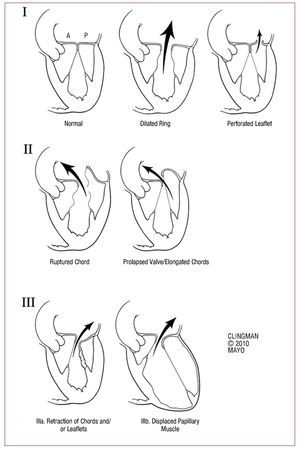
MODERN TREATMENT OF SEVERE CHRONIC ORGANIC MITRAL REGURGITATIONFigure 4 provides a simple algorithm which tries to summarize the complexity of current management of severe MR. When referring a patient with MR, the etiology / mechanism of regurgitation should be determined. There are three basic mechanisms based on the mobility of the leaflets (Figure 5). The first is normal mobility with poor coaptation due to annular dilation or perforation of a leaflet. The second involves excessive mobility (prolapse or ruptured chordae) and the third, decreased mobility (shrinkage due to inflammatory or ischemic disease) (Figure 5).
Figure 5. Mechanisms of mitral regurgitation. I: normal mobility of leaflets with annular dilation that causes a central jet or perforation of a leaflet (eg, cleft mitral valve). II: excessive mobility with ruptured chords or severe prolapse (eg, Barlow disease), causing a jet directed to the opposite side of the affected leaflet. III: restricted mobility due to shrinkage of the subvalvular apparatus (IIIa, eg, rheumatic disease) or displacement of the papillary muscle (ischemic remodeling) that causes apical displacement (tethering) of the leaflet (IIIb). Note that in both cases the jet is aimed at the same side of the affected valve.
Organic MR may be due to a type 1 (eg, cleft mitral valve), type 2 (eg, MR arising from Barlow's disease) or type 3 (eg, rheumatic mitral valve) dysfunction. Functional MR is usually due to a combination of dysfunction 1 and 3.
Mitral regurgitation mediated by the second mechanism is the most susceptible to repair, especially when the posterior leaflet is involved and the ring does not show severe calcification. However, repair in the case of Barlow's disease with severe bileaflet prolapse or prolapse isolated from the anterior leaflet is also now as feasible as in isolated prolapse of the posterior leaflet. Repair is therefore possible in the vast majority of cases of mitral prolapse, when it is performed by experts.21 Once the organic mechanism has been established, it is necessary to definitively confirm that the MR is severe (Table 2). The presence of symptoms, EF <60% or LV ESD ≥40 mm means ventricular dysfunction is manifest and the patient must be referred for salvage surgery (Figure 4). The aim of such surgery is to repair the valve or replace it if repair is not possible. In the absence of manifest ventricular dysfunction, patient risk should be stratified by investigating the presence of risk factors (Figure 4). If risk factors are present (Table 3), the valve should be repaired early, but not before considering the patient's intrinsic factors and factors inherent to surgery: if the patient has a good life expectancy and the valve is repairable, the operation should be performed in a centre which can guarantee a high surgical success rate and low operative mortality (Figure 4). If necessary, the patient should be referred to a specialized center. If there are no such preconditions, "watchful waiting" (monitoring by a cardiologist with an echocardiogram every 6 months) can be implemented for signs echocardiographic and / or symptoms that indicate need for rescue, repair or replacement. If the patient has risk factors (Table 3), we recommend early repair, as there is a risk of short-term progression and prognosis is affected. There is thus a discrepancy between current guidelines and our recommendations, as we believe that finding risk factors should be a type I indication for early repair. If there are no risk factors, 'watchful waiting' is safe and recommended.
ISCHEMIC MITRAL REGURGITATION: A HIGH RISK GROUP
The high risk implied by the ischemic rupture of a papillary muscle is well-known and requires immediate surgery. Operative mortality for papillary muscle rupture has decreased significantly (67% up to 1990 and 9% thereafter), reflecting the frequent use of revascularization and the surgical advances.36 In addition, 5-year survival in patients who survive the initial rupture is identical to that of patients with myocardial infarction without papillary muscle rupture. This highlights the importance of early diagnosis and aggressive surgical treatment when this complication is present.
After myocardial infarction, MR can develop without papillary muscle rupture, as a result of LV remodeling due to apical and inferior displacement of the papillary muscles (Figure 5, IIIb). These then pull on the mitral leaflets causing tenting, with the resultant apical displacement of the coaptation point. The strong negative impact of this 'functional' post-ischemic MR after myocardial infarction has been demonstrated in two studies in which the mere presence of even mild ischemic MR was associated with low survival.37,38 In patients with previous myocardial infarction, MR with an ERO ≥0.2 cm2 is associated with 30% survival at 5 years, compared to 60% in patients without MR. We therefore believe that an ERO >0.2 cm2 or RVol >30 mL in ischemic MR represents severe MR (Figure 4). Comprehensive echocardiographic quantification in these patients is essential to determine the severity of MR, as auscultation does not reveal a significant systolic murmur.39
The timing of surgery in ischemic MR is more complicated than in organic MR. Although repair is possible in most patients, the risk associated with surgery is greater in ischemic MR. Moreover, even if there is symptomatic improvement with surgical correction of dilated and ischemic MR (usually annuloplasty),40 benefits in the long-term survival of these patients have not been observed with correction of MR. However, benefits may exist, especially with repair techniques specifically aimed at resolving the tenting and which are not limited to annuloplasty. Prospective studies with randomization of patients are still required, however.
It has not yet been clarified whether ischemic MR is a cause per se of poor prognosis or whether it is simply a marker of the severity of ischemic heart disease.41 We believe that the degree of ischemic MR is a marker of the severity of heart disease (previous infarction), but also adds an additional volume overload for a weak ventricle. It seems likely that that both factors will give rise to a poor prognosis.
Prosthetic mitral ring annuloplasty can lead to mitral stenosis42 and does not correct the basic problem of displacement of the papillary muscle which results in tenting of the leaflets. Therefore, the reduction of MR with mitral annuloplasty in ischemic MR, although sufficient in some cases of mild MR, is not lasting in many other cases and new techniques are being studied for mitral repair. The concept of repositioning the papillary muscles looks promising and is under development.43
When the mitral valve leaflets are subjected to stress due to tenting, are reactivated embryogenic processes are reactivated that result in cellular activation and transformation of endothelial cells in the interstices resulting in new cellular matrix. All this results in increased valve area and chordal thickening, which can lead to increased area of coaptation of the leaflets.44 This is a breakthrough that promises opportunities in cell therapy for secondary MR. It has recently been shown that surgical extension of the posterior leaflet using bovine pericardium increases the area of coaptation and promises to be a therapeutic possibility with benefits in the short and medium term.45
In dilated cardiomyopathy, it is now possible to more accurately predict the possibility of repair by observing the mobility of the distal aspect of the anterior leaflet. If mobility is preserved, coaptation tends to remain even if the posterior leaflet is immobile after repair. Distal anterior leaflet mobility can be predicted via a simple measurement parameter in the preoperative echocardiogram.46 Progress is therefore being made towards improved functional repair of the MR, with the process of repair being more complex and specific to each patient.
Currently, when surgical revascularization is planned and ischemic MR is mild or moderate (ERO <0.2 cm2), the concomitant correction repair (simple annuloplasty) should be considered (Figure 4). Patients with ERO>20 mm2 should be offered repair (preferable) or mitral valve replacement (Figure 4). When CABG is not considered vital but is an option, the presence of viable myocardium and MR ERO >20 mm2 should lead to combined mitral surgery (repair or replacement) with revascularization, especially if there are symptoms or a history of heart failure.
When bypass surgery is not possible or is not indicated and ischemic MR is ERO >0.2 cm2, the indication for mitral surgery is more restrictive and should be considered only in patients with symptoms of functional class (NYHA) III-IV even with maximal medical therapy, including resynchronization. In these cases, replacement may involve a prohibitively high surgical risk, especially with EF <30% (Figure 4), so repair is essential. Alternatively, cardiac transplantation can be considered in selected patients and may provide optimal results.
PERCUTANEOUS APPROACHES TO MITRAL REGURGITATION
Percutaneous mitral annuloplasty based on implanting a device in the coronary sinus is currently under study. Although the coronary sinus is anatomically located on a superior plane to the mitral ring and there is a real possibility of compromising circumflex coronary flow, results from 48 patients with dilated cardiomyopathy showed that implantation of the device is safe in selected patients and results in increased functional capacity (NYHA).47
Another percutaneous approach to organic or functional MR that originates in the A2 and P2 area uses the E-valve/MitraClip. This is based on the Alfieri suture and creates a double mitral valve orifice. It has been studied in 107 selected patients; the procedure was successful in 74% and 66% of patients were still alive and without a need for mitral surgery at 12 months. MR was <2+.48
CONCLUSIONS
Mitral regurgitation is prevalent in the population as is its progression to impaired LV function due to adverse remodeling. This highlights the serious prognosis associated with the disease in patients who do not receive appropriate treatment.
The detection of subclinical LV dysfunction based on echocardiographic parameters (EF and ESD) should lead to consideration of immediate rescue mitral surgery. The parameters currently used (EF <60% and ESD ≥40 mm) for asymptomatic patients lead to late salvage surgery and should be modified. Similarly, the detection of even minimal symptoms should result in salvage surgery. Stress testing should be used to determine functional capacity in patients with uncertain symptoms.
Not all severe organic MRs are equal. The presence of risk factors in severe MR increases the likelihood of deterioration in cardiac function, development of symptoms, and worse prognosis in the long term, so it is necessary to consider each patient individually. In the absence of symptoms in patients with severe MR organic but no cardiac dysfunction (EF >60%, DTS <40 mm), the presence of risk factors implies treatment with early surgery to repair the valve, if the surgical risk is low and the possibility of repairing the valve is 90%-95%. If necessary, patients should be referred to a specialized center. Proper training of surgeons in mitral repair is essential in order to achieve the best possible survival for patients with severe organic MR and an indication for surgery. National and international collaboration in such training is essential.
Ischemic MR has a worse prognosis, with higher surgical risk. Treatment decisions should be specific to each patient and should take into account the possibility of repair, the surgical risk, and the need for concomitant surgical revascularization.
New percutaneous procedures are being studied which promise to be potentially useful for selected patients.
ABBREVIATIONS
CABG: coronary artery bypass graft
EF: ejection fraction
ERO: effective regurgitant orifice
ESD: end systolic diameter
LV: left ventricle
MR: mitral regurgitation
RVol: regurgitant volume
Correspondence: Dr H.I. Michelena.
Mayo Clinic.
200 First Street SW. Rochester. MN, 55905. United States.
E-mail: michelena.hector@mayo.edu
Received November 15, 2009.
Accepted for publication May 6, 2010.