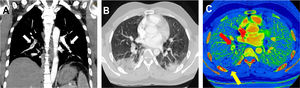
We report the cases of 2 patients from Barcelona, Spain, admitted to the emergency department of our hospital secondary to COVID-19 (formerly known as SARS-CoV-2) pneumonia, confirmed with a real-time reverse-transcription polymerase chain-reaction test1,2; both patients showed respiratory deterioration and elevated serum D-dimer levels. Figure 1 illustrates the case of a 32-year-old man, with no comorbidities or risk factors, admitted to our emergency department on day 14 after symptom onset with dry cough, asthenia, arthromyalgia, fever, and right pleuritic pain. A baseline electrocardiogram showed sinus rhythm, 97 bpm, normal PR interval (120ms) and normal QRS complex (80ms), aQRS 0°. QTc (Friderica) 415 mseg. Echocardiography was not performed but initial physical examination showed systemic blood pressure values of 136/79mmHg, regular rhythm with no murmurs, present and symmetrical distal pulses, and no signs of deep vein thrombosis. Laboratory data showed elevated ferritin levels (615 ng/mL), C-reactive protein (CRP)=3.6mg/dL, and increased interleukin-6 (IL-6) (144.7 pg/mL). Coagulation studies: prothrombin time (PT) 12seconds, international normalized ratio 1.1, partial thromboplastin time (aPTT) 28.2seconds. Lupus anticoagulant testing was positive. Immunoglobulin G and immunoglobulin M anticardiolipin antibodies were also tested with a negative result. D-dimer levels were elevated up to 2460 ug/L and therefore, due to high suspicion of pulmonary thromboembolism, dual-energy pulmonary computed tomography (CT) angiography (CTPA) was performed. CTPA confirmed bilateral thromboembolism associated with multiple opacities compatible with viral pneumonia (figure 1A,B). Iodine map images showed a triangular peripheral pulmonary infarction (figure 1C).
A: computed tomography angiography maximum intensity projection oblique coronal reconstruction image showing filling defects (white arrows) in bilateral segmental and subsegmental branches of pulmonary arteries. B: transverse computed tomography image obtained with lung window settings showing wedge-shaped bilateral opacities with surrounding ground-glass opacities compatible with viral pneumonia. C: iodine map images showing a triangular peripheral area of decreased perfusion (yellow arrow) in the right lower, distal to PE (red arrow) lobe compatible with pulmonary infarction.
The patient received therapy with hydroxychloroquine at a loading dose of 400mg on the first day followed by a maintenance dose of 200mg/d for the next 4 days. Azithromycin 500mg/d for 3 days and enoxaparin 80mg/12h for 10 days were also prescribed. Throughout the admission, the patient showed clinical improvement with no respiratory support requirements, maintaining oxygen saturation levels around 97% to 99% on room air. On the 10th day after admission, 24 days after symptom onset, the patient was discharged with good health status and was asymptomatic. Given the positivity to lupus anticoagulant autoantibodies, thrombophilia testing will be performed in 3 months. A full-dose anticoagulation regimen (80mg/12h) was prescribed for 6 months.
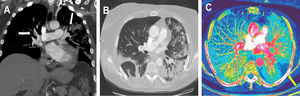
Figure 2 illustrates the case of a 59-year-old woman, with a history of idiopathic hypertension (treated with ramipril 5mg/d) and hypothyroidism (treated with levothyroxine 112μg/d), without other risk factors or comorbidities, admitted to our hospital for 10 days with dry cough, myalgia, and fever. The baseline electrocardiogram showed sinus rhythm, 86 bpm, normal PR interval (131ms) and normal QRS complex (93ms), aQRS 0°. QTc (Friderica) 412ms. Ecocardiography was not performed, but initial physical examination showed systemic blood pressure of 116/78mmHg, regular rhythm, and present and symmetrical distal pulses, without signs of deep vein thrombosis. Laboratory data showed elevated ferritin levels (1127 ng/mL), CRP=9.5mg/dL, and increased serum IL-6 (75,60 pg/mL). Coagulation studies: prothrombin time (PT) 10.7seconds, international normalized ratio 1.09, partial thromboplastin time (aPTT) 33.6seconds. D-dimer at admission was 1320 ug/L. The patient received initial treatment with hydroxychloroquine at a loading dose of 400mg/12h on day 1 followed by a maintenance dose of 200mg/12h for 4 days. She was also prescribed azithromycin 500mg/d for 5 days, anticoagulant prophylaxis with enoxaparin (40mg/d), methylprednisolone 70mg/d for 5 days, and a single intravenous dose of tocilizumab (400mg).
A: computed tomography angiography maximum intensity projection oblique coronal reconstruction image showing filling defects in bilateral segmental and subsegmental branches of pulmonary arteries. B: transverse computed tomography image obtained with lung window settings showing wedge-shaped bilateral opacities with surrounding ground-glass opacities compatible with viral pneumonia. C: iodine map images showing a peripheral, triangular and hypoperfused area in the left lower lobe (yellow arrow), inside the peripheral mnemonic opacities, suggestive of pulmonary infarction.
On day 9 after admission, the patient showed oxygen desaturation and reported chest pain. D-dimer elevation up to 6120 ug/L was observed (previous 1870 ug/L) and therefore, due to high suspicion of pulmonary thromboembolism, CTPA with dual-energy mode was obtained and confirmed a bilateral acute pulmonary thromboembolism associated with bilateral pulmonary opacities compatible with viral pneumonia (figure 2A,B). Iodine map images depicted a peripheral pulmonary infarction (figure 2C). A full anticoagulant regimen with enoxaparin 60mg/12h was added to the treatment from that day until discharge.
Considering the long hospitalization of patients, pulmonary thromboembolic complications are increasing and must be considered in the context of COVID-19 pneumonia.3-5 It is also important to evaluate the possible onset of pulmonary infarcts secondary to pulmonary thromboembolism, which change the patient's management and prognosis. In this clinical scenario, the use of advanced imaging methods, such as dual energy pulmonary angiography, allows differentiation between lung parenchyma affected by COVID-19 pneumonia and ischemic or infarcted areas.
.
We thank fellow health and nonhealth workers who helped us in this study and who are struggling in this global emergency.