COVID-19 overwhelmed the emergency assistance during the winter/spring of 2020. One of its most common manifestations is bilateral pneumonia, which, in its most severe forms, is associated with profound hypoxemia. This presentation is usually treated as a type of acute respiratory distress syndrome (ARDS). Due to association described between right ventricle (RV) dysfunction and ARDS,1 some COVID-19 patients may develop this complication. We present a case in which this reasoning was not followed.
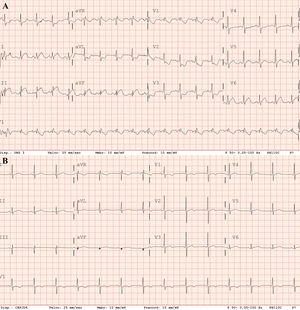
A 61-year-old former male smoker, with a history of hypertension treated with angiotensin-converting enzyme inhibitors, attended the emergency room complaining of dyspnea after a 1-week history of dry cough. His blood pressure was 125/90mmHg, heart rate 136 bpm, respiratory rate 30 bpm, and oxygen saturation (SatO2) <85% on room air. Physical examination showed signs of hypoperfusion and use of accessory respiratory muscles. Therefore, a diagnosis of SARS-CoV-2 was suspected and noninvasive mechanical ventilation was started without response. An electrocardiogram (ECG) showed sinus tachycardia, ST-segment elevation in inferior leads, and ST depression in V2-V4, I and AvL (figure 1A). Thoracic X-ray revealed bilateral pulmonary infiltrates. Echocardiography showed important RV dilatation, apex hypercontractility, RV lateral wall akinesia, and interventricular septal shift due to pressure overload, as well as an estimated pulmonary arterial systolic pressure (PAsP) >60mmHg.
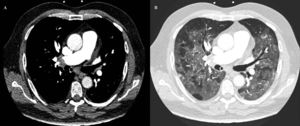
Due to refractory hypoxemia, the patient was intubated and underwent invasive mechanical ventilation. Eventually, persistent hypotension and desaturation developed, requiring crystalloid solution expansion, as well as noradrenaline and dobutamine. Despite the supportive measures, SatO2 showed no improvement. The echocardiogram was repeated, showing a worsening of the RV, as well as an increase in PAsP. At that time, with suspicion of pulmonary embolism (PE), thrombolytic treatment with alteplase was started, according to clinical practice guidelines. The patient started improving progressively, reaching SatO2 90% and hemodynamic stability. Afterward, acute bilateral PE was confirmed by computed tomography angiography (figure 2A) with a finding of diffuse bilateral ground glass opacities in pulmonary parenchyma suggesting an atypical viral infectious etiology (figure 2B). A postreperfusion ECG showed normalization of secondary repolarization changes, with persistence of RV overload (figure 1B). Due to the initial ECG changes, a coronary angiogram was performed showing no significant coronary stenosis.
The patient's SARS-CoV-2 improved during his intensive care unit stay. He tested positive for COVID-19 on reverse transcriptase-polymerase chain reaction. After 4 days of invasive mechanical ventilation, a neurologic examination showed no deficits and weaning was started with good response.
COVID-19 (SARS-CoV-2) challenges both health service resources and diagnosis due to its wide range of complications,2 the most threatening being severe acute respiratory insufficiency. However, there have been reports of other life threatening complications, such as PE due to COVID-19. Through this case, we highlight a therapeutic intervention that could have been hard to justify in different settings. We support urgent fibrinolysis in patients with bilateral lung infiltrates and respiratory symptoms suggestive of infectious etiology, complicated with acute PE.
Acute cor pulmonale is a complication of ARDS patients, particularly those with severe hypoxemia.1 Alveolar collapse, hypoxic pulmonary vasoconstriction and mechanical ventilation increase pulmonary vascular resistance and consequently RV load. Two major differences in contrast with classic ARDS can decrease the risk of RV failure in SARS-CoV-23: a) a blunted hypoxic pulmonary vasoconstriction, and b) a higher lung compliance. Both factors, along with an increased risk of thromboembolic phenomena,4 make PE a highly probable diagnosis when RV failure develops in an infected patient, independently of radiographic characteristics.
The clinical picture was also obscured by an ECG that was highly suggestive of acute myocardial ischemia. Although ST-segment elevation has been described in anteroseptal leads (V1-V4) in patients with PE,5 there are no data on ST elevation in inferior leads in PE. RV overload is the most widely accepted hypothesis but the underlying mechanism remains unclear. Although embolic events in coronary arteries have been described, in our case, the ECG changes were attributed to profound hemodynamic instability and decreased blood oxygen content leading to myocardial hypoperfusion. Our decision to use fibrinolysis was guided mainly by the presence of signs of RV overload, severe pulmonary hypertension with refractory hypoxemia, persistent hypotension, and McConnell's sign, independently of the possibility of concomitant myocardial ischemia. Severe pulmonary hypertension and McConnell's sign are exceptional in RV infarction. This reasoning is consistent with the very low increase in troponin I, no ischemic changes on ECGs and echocardiograms during follow up, and the normal results of coronary angiography.
We would also like to highlight that, in COVID-19 patients with clinical deterioration without a clear explanation, triple-rule-out computed tomography angiography could provide a cost-effective evaluation of the coronary arteries, aorta, pulmonary arteries, and adjacent intrathoracic structures in patients with chest pain and low or intermediate pretest probability. Nonetheless, in our case, this procedure was not indicated due to high pretest probability and low quality of coronary artery images due to tachycardia and hemodynamic instability.6
In conclusion, COVID-19 complications challenge our diagnostic skills. As long as we are able to broaden our knowledge of the physiopathology of this disease we can improve our ability to reduce its associated mortality.
.