Keywords
INTRODUCTION
The anatomic classification of the extension of coronary artery disease to one, two, and three vessels has been used routinely in the prognostic assessment of patients with ischemic heart disease and in the selection of patients for revascularization.1-3 However, this criterion is too simple and not free of limitations. In 1986, Plotnick4 noticed that patients with three-vessel coronary artery disease did not constitute a homogeneous population. He remarked that clinical decisions based only on the number of vessels exemplify a unidimensional approach to a multidimensional problem, and demanded «less anatomy and more physiology» in the assessment of ischemic heart disease. In an attempt to overcome these limitations, different systems for scoring coronariography have been devised,5-10 but subjective assessment of the coronary stenoses continues to be subject to considerable interobserver variability.11-13 This variability can be considerable although quantitative programs are used.14 Topol and Nissen15 warned in 1995 of the excessive concern of cardiologists for «coronary luminology,» in spite of the dissociation often existing between coronariography and clinical manifestations. The use of intracoronary ultrasound is also just a morphometric approach to the study of the coronary arteries.
At present, more credence is conceded to knowledge of the functional repercussion of a specific coronary stenosis that can be studied in the course of catheterization by assessing coronary reserve using pressure guide wires, intracoronary Doppler, myocardial perfusion SPECT, echocardiography, and the conventional electrocardiographic exercise stress test. However, the anatomofunctional correlation of coronary lesions is not always optimal, since different factors, such as the methods used, interpretation criteria, provocation maneuvers, and the patient´s treatment can influence the results of these studies. This motivated Ellestad16 to affirm recently that the time has come to seek a new gold standard for re-evaluating the effectiveness of noninvasive tests.
It is interesting that the invasive techniques used for the assessment of coronary physiology have used as a gold standard noninvasive functional studies such as tomographic radionuclide scans of myocardial perfusion and stress echography, which are not exactly characterized as techniques that allow absolute quantification of coronary flow. The only noninvasive technique that allows quantitative assessment is positron-emission tomography, but it is highly complex and expensive, so its use is strictly limited even in centers that are equipped with the technique. It can be affirmed that the cardiologist currently has more possibilities for measuring anatomic parameters invasively (quantitative coronariography, intravascular ultrasound) than for quantifying absolutely functional parameters by invasive (intravascular pressure guide wire and Doppler) and noninvasive means (tomographic perfusion radionuclide scan, stress echocardiography, contrast echocardiography). As will be seen later, the values obtained from the studies of functional parameters are only semiquantitative, the result of comparing the most affected coronary regions with less affected or normal areas.
However, in clinical practice the cardiologist often has the problem of assessing the true importance of a single stenosis that is considered moderate in the coronariography, or as a preliminary step to considering partial or total revascularization in a coronary patient with multivessel disease. The practice of percutaneous coronary intervention (angioplasty or stent) has increased enormously in the last decade. Initially, percutaneous revascularization techniques were not performed during diagnostic catheterization, but at the present, in order to reduce the aggressiveness of the procedure for the patient, avoid vascular complications, and reduce costs, revascularization is increasingly performed during the same procedure. Therefore, it is not uncommon that the cardiologist ordering a diagnostic coronariography indicates on the order that revascularization may be performed, depending on the result of coronariography. Incomplete percutaneous revascularization has been recognized as a reasonable strategy in many patients with multivessel disease, and it is known that total revascularization is performed in only 10% of these patients using percutaneous coronary interventionist techniques (angioplasty, stent). It is in such cases that previous knowledge of the location of the most functionally stenotic coronary artery, the so-called culprit lesion or the lesion responsible for the clinical manifestations of the patient or the most severe ischemia, is extremely important .
In this article, after a brief description of the physiology of the coronary circulation, the current status of invasive and noninvasive studies that can help cardiologists to make functional assessments will be described.
PHYSIOLOGY OF CORONARY CIRCULATION
Before approaching in detail the different techniques for functionally assessing the coronary circulation, it is advisable to briefly review some physiological facts that are fundamental for correctly interpreting the results of these studies. The coronary circulation it common described in terms of analogies with simple electrical or hydraulic circuits. In fact, these models are far from capable of integrating the complexity of the phenomena that occur during the cardiac cycle.
Heart function is highly dependent on the maintenance and modulation of coronary blood flow. The myocardium, particularly the subendocardium, is the tissue with the highest baseline aerobic demands in the body (8-10 ml O2/min/100 g vs 0.15 ml O2/min/100 g in the skeletal muscle). The three main determinants of this demand are wall stress, inotropic state, and heart rate.17,18
Coronary blood flow is influenced by extravascular compression. Throughout the cardiac cycle, variations in intramyocardial and intracavitary pressure modify coronary vascular resistance drastically.19 Coronary blood flow is modulated by variations in the resistance of the vascular bed. Whereas blood flow is predominantly diastolic in the left coronary artery, in the right coronary artery there is also systolic blood flow due to the low extravascular compression exerted by the right ventricle and atria.19,20
During cardiac systole, retrograde flow takes place in the coronary arteries and anterograde flow in the coronary veins. As a result of the extravascular compression resulting from systole, the microcirculatory and intramural vascular bed empties in two directions, retrogradely in the arterial direction and anterogradely in the venous direction, which causes a 180° phase difference in the flow in the coronary arteries and venous sinus.17,21-23
During diastole, part of the anterograde flow fills the intramyocardial microcirculatory bed. Although coronary blood flow is fundamentally diastolic, during protodiastole epicardial blood flow and myocardial perfusion dissociate because the blood flow dissipates in a capacitance phenomenon, as filling the intramural branches and microcirculation milked during systole are filled.21,24
In baseline circumstances, the relation between blood pressure and flow in the coronary arteries is not linear. If the only situation considered from now on is analogous to the mid end-diastole, that is to say, a situation in which extravascular compression is minimal and constant and there are no variations in coronary conductance, coronary blood flow remains stable over a broad range of pressures. This phenomenon receives the name of coronary self-regulation and is the result of intrinsic myogenic tone, a response by the smooth muscle cells of the coronary arterioles to pressure variations.17,21,25-27 Coronary self-regulation is only effective within the range of pressures indicated: when the perfusion pressure falls below this pressure, coronary blood flow decreases.
During maximum coronary hyperemia, the relation between coronary blood pressure and flow is linear. In contrast to the situation just described, complete vasodilation of resistance vessels induced by a maximum physiological or pharmacological hyperemic stimulus (increased myocardial metabolism) establishes a fixed relation between coronary perfusion pressure and blood flow. The slope of this relation is influenced by the resistance of the system: the lower this slope (conductance) is, the greater the resistance of the system will be.21,28 The pressure-flow relation during maximum hyperemia constitutes a ceiling of expected coronary flow values for different perfusion pressures.29
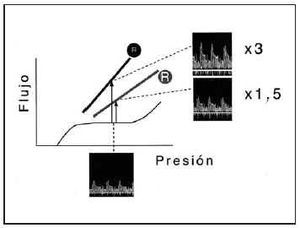
The increase in blood flow from the self-regulation to the maximum hyperemia situation constitutes an indicator of the functional state of the coronary system. This concept constitutes the coronary flow reserve, a functional indicator of the state of coronary circulation that is widely used in diagnostic techniques.17,21,25-27,29 In figures 1 through 3 this principle is illustrated schematically. It is important to remember that in normal conditions the coronary reserve is transmurally heterogeneous: in the subendocardium, the coronary reserve is smaller because there is a greater baseline degree of arteriolar vasodilation as a result of greater subendocardial metabolic requirements.18
Fig. 1. Application of the relation between intracoronary pressure and flow to the diagnostic study of coronary circulation. The basic concept of coronary reserve is shown in this figure. In baseline conditions, the relation between pressure and flow are not linear, but are characterized by a broad plateau of pressures for which coronary flow remains constant, a phenomenon modulated by arteriolar resistance. Nevertheless, during maximum hyperemia induced by myocardial oxygen demand or the administration of vasodilator drugs, this relation is linear (solid lines). The slope of this relation expresses coronary resistance (R). A situation is considered physiological when resistance is normal (small R). The induction of hyperemia means that, for a given pressure, coronary flow increases 3 times over baseline values (arrow). Nevertheless, an increase in resistance (large R) resulting from epicardial stenosis or microcirculatory dysfunction, and manifested as a less pronounced slope in the pressure-flow relation, is associated with a smaller increase in flow in relation to baseline values for the same pressure (1.5 times the baseline flow value). The intracoronary velocity tracings obtained with a Doppler guide wire make it possible to obtain, in accordance with this principle, coronary flow velocity reserve (CFVR).
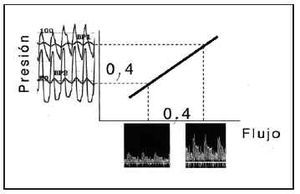
Fig. 2. The existing linear relation between pressure and flow during hyperemia makes it possible to infer, in accordance with pressure measurements, the degree of limitation to flow caused by epicardial stenosis. The graph shows the flows corresponding to pressures BP1 and BP2, which have a fixed relation dictated by the slope of the relation between pressure and flow during hyperemia. When the difference between these pressures results from the hemodynamic effect of an epicardial stenosis (transtenotic gradient in hyperemia), it is inferred that the percentage drop in flow faithfully reflects the percentage drop in pressure through the same stenosis (0.4 in both). Intracoronary pressure recordings obtained by a pressure guide wire thus make it possible to obtain the myocardial fractional flow reserve, which in this case indicates that the coronary blood flow received by the myocardium dependent on the vessel is only 40% of the flow that would be received in the absence of stenosis.
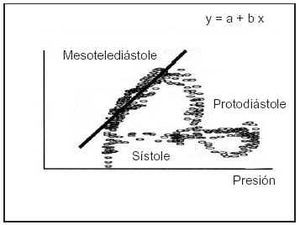
Fig. 3. The slope of the pressure/flow relation in hyperemia, an index of coronary resistance, can be directly estimated. To do so, it is necessary to have instantaneous information on intracoronary pressure and flow. Using only data collected in mid end-diastole, regression analysis can be used to establish the slope (b) of this relation. This index receives the name of instantaneous hyperemic diastolic pressure-flow slope (i-HDPFS).
The presence of epicardial stenosis causes a loss of energy associated with blood flow that is expressed as a fall in effective perfusion pressure. Stenoses cause two types of resistance, one related with friction (f) and the other related with turbulence and the dispersion of blood flow after passing the stenosis (s). The transtenotic pressure gradient, ΔP, has a nonlinear relation with the f and s components and flow, Q, in accordance with the expression ΔP=fQ+sQ2. The resistive components of friction and turbulence are influenced by characteristics of the blood (viscosity and density) and the geometry of the stenosis (reduction of luminal area, length, and the inflow and outflow angles).26,29,30 The complex interrelation between these factors contrasts with the simplicity of the indices of angiographic severity commonly used in clinical practice (e.g., the percentage of luminal diameter), and illustrates the limitations of angiography for evaluating the functional repercussions of stenosis.31
Coronary self-regulation compensates for the fall in blood pressure caused by stenosis in order to maintain constant coronary blood flow. The mechanism of self-regulation, which in physiological conditions adjusts microcirculatory resistance to myocardial energy requirements, has a chronic compensatory function in response to the fall in intracoronary pressure secondary to stenosis. As the stenosis increases in severity, sustained arteriolar vasodilation increasingly compromises the self-regulatory function to maintain an adequate myocardial blood flow. In other words, the compensatory function of coronary self-regulation in a stenotic vessel is at the expense of reducing coronary reserve. The compromised coronary reserve is first evident in the subendocardium, where baseline arteriolar vasodilation is greater due to higher energy demands.
The hemodynamic effect of a stenosis is manifested in the smaller slope of the pressure-flow relation. The diagnostic utility of the coronary reserve concept derives from this finding. The potential increase in coronary blood flow from a given pressure at baseline to maximum hyperemia (that is, the coronary reserve) decreases in the presence of stenosis. This effect is quantifiable in absolute terms if measurements obtained at baseline and in hyperemia are available. In addition, because adjacent vascular beds have a conserved coronary reserve, the induction of maximum hyperemia tends to increase the heterogeneity of myocardial perfusion, a phenomenon that constitutes the basis for different diagnostic techniques. In this sense, pure arterial vasodilators (adenosine, papaverine, dipyridamole) administered systemically, enhance the heterogeneity of regional and transmural myocardial perfusion (inducing the theft phenomenon when the vessel of the epicardial layers dilate). Dobutamine exhausts the coronary reserve in the stenotic vessel as a result of metabolically increasing myocardial demand (it increases contractility and myocardial oxygen consumption more than physical exercise) and specifically inducing vasodilation of the microcirculation (doses of 30-40 µg/kg/min produce overall coronary vasodilation similar to that of adenosine administered systemically).32 Finally, physical exercise is the most physiological stimulus, since it combines metabolic stimuli with neural modulation of the coronary circulation.
Microcirculatory dysfunction is also manifested the smaller slope of the pressure-flow relation. This is important for the correct interpretation of diagnostic techniques based on coronary reserve, and explains the development of techniques for the specific assessment of epicardial and microcirculatory resistance.33-36 Many clinical entities that cause remodeling of the coronary microcirculation also participate in the development of epicardial stenoses, such as diabetes mellitus, arterial hypertension, smoking, and hypercholesterolemia, as well as heart transplant vasculopathy.26,36 Likewise, microcirculatory resistance can increase in relation to alpha-adrenergic stimulation (e.g., in relation to physical exercise or mental stress),37 during acute myocardial ischemia,38 or as a result of microembolization (platelet or thrombotic aggregates, particles resulting from rotational atherectomy).39
Another important observation for diagnostic techniques is that microcirculatory dysfunction reduces the gradient of transtenotic pressure in an epicardial stenosis that, as discussed above, is dependent on coronary blood flow. This phenomenon can lead to an incorrect interpretation of diagnostic tests that are based on the Bernouilli formula or measurement of the translesional gradient.35,40 This again explains the importance of tests that allow independent assessment of the severity of an epicardial stenosis and the state of microcirculation.
INVASIVE METHODS FOR THE PHYSIOLOGICAL ASSESSMENT OF CORONARY CIRCULATION
The techniques of intracoronary physiological assessment described below arise from the possibilities, made available by technological advances, to make the same observations used to clarify the pathophysiology of ischemic heart disease experimentally for clinical purposes, and to develop noninvasive techniques for detecting ischemia. By virtue of its invasive character, intracoronary physiological assessments are always made with previous knowledge of the coronary anatomy and in a highly selective way. Studies are made of individual vessels or the stenosis itself, which allows the assessment of patients with single or multivessel disease. Since these interventions are percutaneous, their results contribute to decision-making and the assessment of the final results during such interventions.
General aspects of physiological assessment in the hemodynamics laboratory
Although various invasive techniques have been used for the physiological assessment of coronary circulation in hemodynamics laboratories,41 the instruments most used at present are very small caliber solid guide wires equipped with speed or pressure sensors.35 Guide wires with velocity sensors provide reliable information about the mean speed of coronary blood flow, based on spectral analysis of the radiofrequency signal obtained. Guide wires equipped with pressure sensors provide measurements of intracoronary pressure with a high frequency response. In both cases, in contrast with previously used techniques, the low profile of guide wires (0.16 mm²) produces negligible interference with the measurements obtained. Doppler guide wires allow good quality recordings to be obtained at baseline and during hyperemia in most cases. Occasionally, the increase in blood flow with the induction of hyperemia can change the position of the guide wire, causing signal loss and making it necessary to reposition the guide wire. In contrast, pressure guide wires do not have to be positioned and provide stable pressure signals that do not change during the induction of hyperemia. Measurements of intracoronary pressure are combined with those of aortic pressure obtained with the catheter guide wire.
Many of the techniques that will be describe require pharmacological induction of maximum coronary hyperemia. The agents most often used are adenosine (or adenosine triphosphate) and papaverine. Adenosine can be administered by the intracoronary (20-40 µg boli) or intravenous route (140-160 µg/kg/ min), although the intravenous route is preferable for the purpose of guaranteeing complete, sustained hyperemia during measurements. It must be used with caution in patients with bronchial hyperreactivity, cardiac conduction disorders, and severe kidney failure. Its effect can be antagonized by concomitant xanthine treatment. Papaverine administered in an intracoronary bolus (12 mg in the left coronary and 8 mg in the right) provides a stable maximum hyperemia lasting approximately 1 min. Finally, although it is not generally used in calculations of coronary reserve, the administration of maximum doses of dobutamine (30-40 µg/kg/min) is also associated with maximum coronary hyperemia.32
Coronary reserve indices
Coronary flow velocity reserve
Doppler guide wires make it possible to calculate an index equivalent to classic, or volumetric, coronary reserve, the coronary flow velocity reserve (CFVR), which is the ratio between intracoronary mean velocity in baseline conditions and after pharmacological induction of maximum hyperemia. CFVR does not discriminate between the effect on coronary reserve of epicardial stenoses or microcirculatory disorders. Its usefulness in the hemodynamic assessment of epicardial stenoses has been studied widely. The most relevant papers in this field are listed in Table 1, with the type of test used to detect ischemia used as a reference.42-49 The cutoff point for determining the hemodynamic relevance of these studies varies, with CFVR <2 generally being accepted as indicative of abnormal coronary reserve. The main limitation of the concept of CFVR is its dependency on values obtained with respect to the baseline flow velocity, which can be modified by many factors,34,35,50 including sex, blood pressure, and heart rate. For this reason, a model has been proposed to adjust CFVR measurements to the baseline speed and age of the patient, a concept designated corrected coronary flow velocity reserve.51
Relative coronary flow velocity reserve
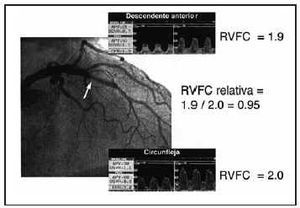
As mentioned, coronary reserve and, therefore, CFVR provide combined information on the epicardial (stenosis) and microcirculatory components of the vessel studied. The potential coexistence of both anomalies can lead to problems in clinical decision-making.34,35 The concept of relative coronary flow velocity reserve (CFVR-r) provides a solution for this problem.52 CFVR-r is the ratio between the CFVR obtained in a stenotic vessel and CFVR in a non-stenotic reference vessel (Figure 4). CFVR-r can have a maximum value of 1, corresponding to the complete absence of hemodynamic relevance in the problem stenosis. Below 0.60-0.65 (Table 1), the problem stenosis would be considered hemodynamically significant regardless of the presence of microvascular dysfunction.47,49 This technique offers the advantage of providing simultaneous information on the state of the microcirculation (CFVR<2 in the reference vessel indicates that the subject has microcirculatory dysfunction). The limitations of the method are the assumption that microcirculation presents a similar degree of impairment in the two territories in which CFVR measurements are made and the need for a non-stenotic reference vessel, which impedes its application in patients with multivessel disease.
Fig. 4. Usefulness of the fractional flow reserve in a diabetic patient with angiographically mild restenosis (arrow) in a stent previously implanted in the anterior descending branch and persistent angina. Noninvasive tests to detect ischemia detection were inconclusive. The coronary flow velocity reserve (CFVR) in the problem vessel is abnormal (1.9), which can lead to the conclusion that the restenosis is hemodynamically significant. Nevertheless, CFVR in the circumflex branch, without angiographically evident stenosis, demonstrates a borderline value with normality that suggests the presence of microvascular dysfunction. The ratio between the two CFVR vales (relative CFVR) permits normalization of the results of the stenotic vessel for the documented microcirculatory substrate in the reference vessel, and demonstrates a normal value (>0.65). In the context of the patient´s microvascular impairment, revascularization of the restenosis indicated would contribute, in the best of cases, only 5% more flow in the anterior descending branch and for that reason should not be performed.
Myocardial fractional flow reserve
Information can be obtained relative to the coronary flow reserve in accordance with intracossure measurements. The principle on which this technique is based derives from the pressure-flow relation in the coronary tree. This relation is linear during hyperemia, so it is evident that the proportion between two intracoronary pressures is identical to the proportion between the two coronary flows corresponding to these pressures. If this concept is applied to pressures proximal (Pa) and distal (Pd) to a stenosis, we will be able to calculate the percentage fall in coronary flow caused by the stenosis: For example, Pd/Pa ratio=0.5 will be interpreted as a 50% reduction in coronary blood flow caused by the stenosis in relation to a non-stenotic situation (in which case there would be no difference between Pa and Pd, and Pd/Pa=1). The Pd/Pa ratio obtained during maximum hyperemia constitutes the myocardial fractional flow reserve (FFR) (Figure 5).53-58 Pijls et al54 found that an FFR value of 0.75 has an elevated sensitivity and specificity for the identification of coronary stenoses associated with inducible ischemia. This cutoff point is based on other studies48,49 summarized in Table 1. A modification of FFR is the diastolic FFR, which is characterized by using the ratio of pressures obtained only during diastole.48 This technique, aside from demonstrating the highest sensitivity for the detection of inducible ischemia, can potentially avoid the interference of systolic phenomena that affect calculations based on mean pressures.59,60
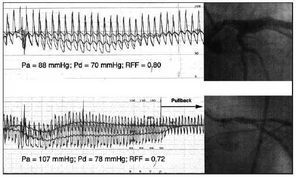
Fig. 5. Application of the fractional flow reserve (FFR) to the assessment of two stenoses of the left common trunk. After the administration of adenosine, an evident increase occurs in the pressure gradient through the stenosis in the common trunk as a result of increased flow. In spite of having a similar degree of angiographic severity, FFR was different in both cases, and coronary revascularization was considered necessary in the case shown in the lower panel, which presented a value <0.75. Observe the persistence of a residual pressure gradient in this case, which disappears when the guide is pulled back through the stenosis, thus confirming the persistence of guide calibration.
Pa indicates aortic pressure; Pd, pressure distal to the stenosis.
FFR has characteristics that deserve detailed analysis. In the first place, it is a specific technique for the hemodynamic assessment of stenoses, so it cannot be applied to coronary arteries without epicardial stenosis. The information that FFR provides is relative to a theoretical hemodynamic situation in which the stenosis to be studied does not exist, a characteristic that makes it especially useful for assessing the need for percutaneous revascularization. FFR evaluation provides combined information on anterograde flow through the stenosis and on collateral circulation in the myocardial territory dependent on the artery to be studied: in vessels with important collateral support, the relative effect of stenosis on the perfusion of the territory is smaller and FFR values are higher. Unlike CFVR, FFR provides very similar values under different hemodynamic conditions.61 Finally, although it does not contribute information on the state of coronary microcirculation, FFR measurements are dependent on the state of the microcirculation. Since the transtenotic pressure gradient is a function of coronary flow, attenuation of the hyperemic response by microcirculatory impairment will be associated with higher FFR values than those obtained in cases of normal microcirculation. From a practical viewpoint, this may not constitute a limitation of the technique: the finding of a normal FFR value in a stenosis with important underlying microcirculatory involvement would make it possible to anticipate a small percentage increment in myocardial blood flow if this vessel is revascularized.35
Methods not based on the concept of coronary reserve: slope of the diastolic pressure-flow relation in hyperemia (coronary conductance) and coronary resistance
In the introduction to the elementary principles of coronary physiology, the relation between coronary blood pressure and flow is emphasized as one of the best ways to approach coronary function. This principle was developed by Mancini et al28 to be used as a diagnostic application designated as the instantaneous hyperemic diastolic pressure-flow slope (i-HDPFS) using only measurements obtained in mid end-diastole. As commented above (Figure 3), during protodiastole there is no relation between pressure and flow due to the capacitance of intramyocardial vessels and microcirculation. Experimental studies have shown a good correlation between the severity of epicardial stenoses and the degree of subendocardial conductance measured with radiospheres. The method has been adapted for clinical use with guide wires fitted with pressure microsensors and flow velocity by Mario et al.62 The principle of i-HDPFS is similar to that of the hemipressure time used in the assessment of mitral stenosis with Doppler echocardiography. It has the advantage of not needing baseline reference measurements because data are collected only during maximum hyperemia. The main limitation of i-HDPFS is the technical difficulty of obtaining the measurement. There are no commercial systems for the measurement of this variable, and in clinical studies, the poor quality of the Doppler signal has made it impossible to perform this calculation in 20% of cases. Controversy exists regarding its dependence on changes in preload, heart rate, and cardiac contractility.61,63
A second approach is to calculate coronary resistance from mean values of coronary pressure and flow velocity, expressed in absolute terms38 or as a ratio between resistance in baseline conditions and hyperemia.64 In spite of their limited diffusion, these techniques have demonstrated their potential for identifying histological changes in microcirculation64,65 and for observations about their role in different coronary syndromes.38,66
A growing amount of info rmation is available on the safety not only of intracoronary maneuvers with pressure and flow guide wires,67 but also on decision-making about revascularization based on intracoronary physiological data obtained with these techniques.68-79
NONINVASIVE METHODS FOR THE PHYSIOLOGICAL ASSESSMENT OF CORONARY CIRCULATION
As discussed in the previous section, the techniques of intracoronary physiological assessment described have been validated by noninvasive functional studies of the coronary circulation: myocardial tomographic radionuclide perfusion scanning (SPECT: single-photon emission computed tomography) and echocardiography. In the hospital setting, half of the indications for noninvasive studies are requested for the functional assessment of a specific coronary stenosis or for the detection of the culprit lesion, after practicing diagnostic coronariography, which indicates that in many cases this last study alone is not sufficient to make an integral assessment of coronary patients.
Radionuclide study of myocardial perfusion
The most recent innovation in nuclear cardiology is positron-emission tomography (PET). This technique uses isotopes with a very short mean life (min/s) and high energy (511 KeV), which is why tissue attenuation is minimal. PET allows the quantitative and noninvasive determination of coronary blood flow80-84 in an exact way. The isotopes most used for the absolute quantification of coronary blood flow are 13N-ammonium and 82Rb. A good correlation has been observed between coronary blood flow determined by radionuclide scan and by microspheres. On the other hand, PET can be used to assess the viability of the myocardium by the combined use of metabolic radiomarkers like 18F-FDG, whose uptake depends on cellular integrity, and perfusion radiomarkers like 13N-ammonium or 82Rb. Clinical experience with PET in cardiology is limited because it is an expensive technique, of limited availability, whose diagnostic performance remains to be determined.
For these reasons, the assessment of myocardial perfusion in clinical practice is usually made by myocardial perfusion scan using single-photon emission computed tomography, or SPECT, which is a technique with which broad experience is available (Figure 6). Myocardial perfusion studies were initiated in the 1970s using thallium chloride (201TlCl) as the radiomarker. Thallium-201 is a monovalent cation of behavior similar to the potassium that penetrates the cell by active uptake (Na pump) and passive diffusion. Myocardial uptake is proportional to coronary flow and peaks at approximately 20 min. Once the peak value is reached, so-called redistribution takes place, a process by which intra and extracellular concentrations balance out and 201Tl circulates from high-uptake to low-uptake zones. This means that the post-stress study must be acquired quickly (within 10 min of radiomarker administration) and before redistribution begins.
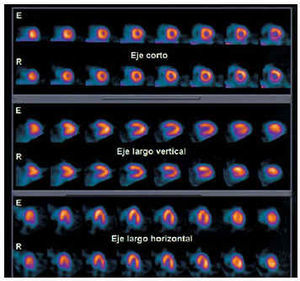
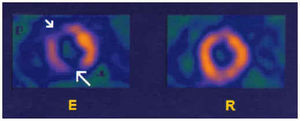
Fig. 6. Tomographic short-axis, vertical long-axis, and horizontal long-axis sections, with effort (E) and rest (R), corresponding to myocardial perfusion SPECT with 99mTc-tetrofosmin in a healthy subject. Note the homogeneous distribution of the radionuclide marker in all the segments of the left ventricle.
In addition to 201Tl, there are currently two other perfusion radionuclide markers available for clinical use: 99mTc-sestamibi (MIBI: methoxy-isobutyl-isonitryl) and 99mTc-tetrofosmin. These agents have different advantages over 201Tl. Unlike 201Tl, they penetrate the cell by passive diffusion and do not present appreciable redistribution. This means that they must be administered in two doses, one for the stress study and the other for the study at rest. Although the efficiency of extraction is greater for 201Tl than for technetium-labeled markers,85 the use of Tl-labeled markers is usually preferred because they can be administered at higher doses and require a shorter examination time. Consequently, they yield images with a greater count density and allow synchronized studies to be obtained without increasing the irradiation of the patient. After intravenous administration, they are distributed by the organism in proportion to regional blood flow and cardiac activity is proportional to coronary flow and to myocardiocyte integrity. For that reason, they have also been used in the diagnosis of myocardial viability.86,87 The utility of these radionuclide markers to evaluate myocardial perfusion has been widely demonstrated and it can be affirmed that there is a good correlation between the decrease in coronary blood flow during stress and the severity of defects in the radionuclide scan.88 Other markers are under study, but not available commercially. They have characteristics similar to those mentioned, for instance, 99mTc-teboroxime,89 Q1290 and, more recently, 99mTc-NOET91 and agonists of the adenosine-A2A receptors.92 There are various protocols for the acquisition of SPECT (stress-rest in one day, two days, etc.) and single detector and multidetector (with two or three detector heads) gammacameras.93,94 The response of the heart to stress can be evaluated with the effort stress test using a treadmill or bicycle, or pharmacological stress: dipyridamole, adenosine and dobutamine, basically. The acquisition of SPECT synchronized with the ECG has been introduced recently (gated-SPECT). This technique consists of synchronizing the onset of acquisition with the R-wave signal in the ECG and dividing the cardiac cycle into 8 or 16 segments. Gated-SPECT enables the simultaneous assessment of coronary perfusion and cardiac function (Figure 7), a good correlation having been demonstrated between the ejection fraction determined by gated-SPECT and that determined by echocardiography95,96 and contrast ventriculography.97 The combined assessment of myocardial thickening and contractility provided by gated-SPECT helps to differentiate defects due to artifacts from those due to ischemia, as well as the myocardial contractile reserve by means of low-dose dobutamine stimulation.98 The latest innovation in this context has been the correction of attenuation, a method for correcting the attenuation of tissues surrounding the heart.99,100
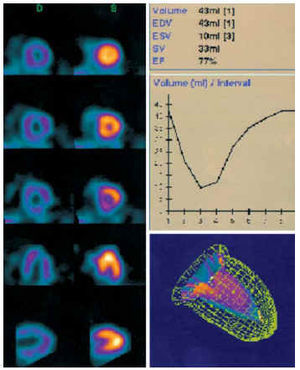
Fig. 7. Normal gated-SPECT corresponding to the study shown in Figure 4. On the left, images in diastole (D) and systole (S) of the short axis, horizontal long axis, and vertical long axis. On the right, left ventricular volume-time curve with the calculation of the ejection fraction and a three-dimensional image of the ventricular contours in end-diastole and end-systole. EDV indicates end-diastolic volume; ESV, end-systolic volume; SV, systolic volume; EF, ejection fraction.
SPECT allows the study of myocardial perfusion by assessing the homogeneity/heterogeneity of the distribution of the radionuclide marker in the myocardium. In the healthy subject, the images obtained post-stress or at rest demonstrate homogeneous activity in the left ventricular myocardium. Any zone of hypoactivity must be considered pathological and must be assessed, especially the changes observed between the image obtained in stress and at rest (Figure 8). When assessing the precision of SPECT for the diagnosis of coronary artery disease, it has been observed that there are no significant differences in results according to the sex or age of the patients, and that the sensitivity and specificity values are about 90-95%.101-103 It is necessary to take into account that are diverse non-atherosclerotic causes that can give rise to perfusion defects: the X syndrome, coronary spasm, coronary ectasia, coronary fistula, and hypertrophic cardiomyopathies.104-106
Fig. 8. Images of myocardial perfusion SPECT with 99mTc-tetrofosmin (short axis) corresponding to a patient with disease of the descending anterior artery alone. A severe defect (arrow) can be seen with effort (R) that is reversible at rest (R).
Diagnosis of multivessel disease
The diagnosis of multivessel disease by means of the radionuclide perfusion scan is based principally on the detection of reversible perfusion defects in more than one coronary region (Figure 9) and other indirect signs, such as transitory ischemic left ventricular dilation and post-effort pulmonary radionuclide uptake. The association of these indicators of severe ischemia in the radionuclide perfusion scan with signs of severity in the electrocardiogram improves the results of both tests considered separately.107-109 After reviewing thousands of patients included in studies in which SPECT and effort or pharmacological echography was performed, it has been concluded that effort SPECT is more sensitive but less specific than stress echography for the diagnosis of coronary artery disease.110,111
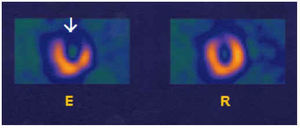
Fig. 9. Images of myocardial perfusion SPECT with 99mTc-tetrofosmin (short axis) in a patient with disease of the anterior descending coronary (AD) and right coronary arteries (RC). The most severe defect (large arrow) corresponds to the lower region. In the anterior region (small arrow) a less severe reversible perfusion defect can be observed. E indicates effort; R, rest.
Assessment of the myocardium at risk
The term «myocardium at risk» is generally used to define the extension of the myocardium threatened by diseased coronary arteries. However, in fact it should refer to the amount of myocardium that could be infarcted if the most stenotic coronary artery was occluded.112 Correlations between different quantitative tomographic radionuclide scanning methods for the assessment of the extension of perfusion defects are very acceptable, as demonstrate by the results of Ceriani et al,113 which compared three quantitative methods for assessing the myocardium at risk by means of SPECT.114-116 The correlations between tomographic radionuclide quantification and different coronariographic qualifications of the entire myocardium at risk are significant although not optimal.117-120 They improve progressively as the coronariographic score becomes more complex (r=0.48 for the Califf method, r=0.59 for the modified Gensini method, and r=0.65 for a coronariographic score in which the presence of collateral circulation is also assessed).117
In coronariography, the role of collateral circulation can be underestimated.120-122 In fact, the best correlations are obtained with the coronariographic score that takes into account the existence of collateral circulation in cases in which an occluded coronary artery exists. Although it is known that not always is the most stenotic artery responsible for future infarction, it would be more logical to use the term myocardium at risk to define the extension of the myocardium that can be infarcted if the coronary artery with the most severe lesion were to be occluded.121-124 The best correlation (r=0.85) between the myocardium at risk obtained by SPECT and that determined by coronariography (considering circulation collateral) is obtained when assessing the culprit lesion.117 It is logical that this is the case, considering that tomographic radionuclide perfusion does not allow absolute quantification of the coronary flow, offering information only about the most hypoperfused region with respect to the least hypoperfused region.
Detection of the culprit lesion
Myocardial radionuclide perfusion scan is very useful in indicating partial revascularization procedures in patients with chronic coronary arterial disease,125-127 since in these cases what we aim to detect is the coronary stenosis that induces ischemia, the so-called «culprit lesion». This term is used as a synonym for the coronary lesion that causes symptoms in patients with ischemic heart disease.128,129 Some authors have relied on coronariography129 and others on radionuclide perfusion scan128 to detect the culprit lesion before performing a partial revascularization. The first possibility occurs generally in patients with unstable angina, and the second in stabilized patients.
The myocardial region with the greatest hypoperfusion in stress is assessed better in short-axis sections, because these images visualize in combined form the four basic regions of the left ventricular myocardium (anterior, septal, inferior, and lateral). In these images, the relative perfusion of these regions can be compared and the most severely affected region determined with respect to the radionuclide scan (Figure 9). The assessment of myocardial ischemia by radionuclide perfusion scan correlates well with CFVR, relative CFVR, FFR, and diastolic FFR (Table 1). Consequently, in cases in which the significance of a certain coronary stenosis is inconclusive,130,131 perfusion tomographic radionuclide scan can be very useful for assessing the severity of ischemia.
The agreement between coronariography and tomographic radionuclide scan for the diagnosis of the culprit lesion is approximately 84%, being higher for the right coronary (91%) and anterior descending coronary (79%) than for the circumflex (62%). Discordant cases usually correspond to cases in which the tomographic radionuclide scan has problems in the assigning ischemia to the territory of the right coronary or circumflex, and to cases of three-vessel disease with stenosis of similar severity of, at least, two of the three affected arteries.117 Among other factors that can help to explain these discrepancies is the method used for to quantify the radionuclide scan132-135 and coronariography,136-139 the presence of collateral circulation,140-142 type of stress used to produce ischemia,143,144 peak oxygen consumption, and myocardial consumption of oxygen reached during the test145 and treatment of the patient.146,147
The extreme culprit lesion case, from a coronariographic vantage point, is the occluded artery. The fact that it is not uncommon to observe occluded arteries in coronariographic studies of patients without a history of myocardial infarction, as well as different degrees of uptake defect severity in territories corresponding to occluded arteries, illustrates the disparity that can exist between a basically anatomic test like coronariography and a functional test like tomographic myocardial radionuclide perfusion scan.148 From the vantage point of clinical care, it has been observed that the contribution of SPECT and coronariography to the therapeutic decision adopted by the clinical cardiologist in 85% of cases coincides with the severity of the disease and, therefore, conditions the attitude (conservative or revascularization) to be adopted.149
Echocardiography for the diagnosis of myocardial ischemia
Echocardiography is very sensitive for the diagnosis of acute ischemia, aided by improvement of imaging in the latest generation of equipment, particularly the development of second harmonic imaging,150 which allows a better definition of the endocardial margin. The main advantages of echocardiography with respect to other techniques for investigating ischemia are its low cost, broad availability, absence of radiation, and the possibility of evaluating associated valve dysfunction.151,152 Its main limitation derives from the difficulty of making a quantitative analysis. However, this can now be palliated, in part, with new techniques like Doppler tissue studies,153-155 the measurement of tissue deformation (strain),156 and the omnidirectional M-mode.157 Another limitation is its broad interobserver variability, which has been observed in studies in which the second harmonic was not yet in use.158
Stress echocardiography
The development of stress echocardiography has also been facilitated by second harmonic imaging, in addition to the availability of software that allows the comparison a posteriori of digitized baseline and stress images. Stress echocardiography has two major clinical applications: the diagnosis of myocardial ischemia and myocardial viability. Provocation maneuvers have been divided by the mode of triggering ischemia. Thus, exercise, dobutamine, and electrical stimulation increase myocardial oxygen demand, and ergonovine reduces demand. Vasodilator drugs like dipyridamole and adenosine produce ischemia by «coronary theft» mechanisms.159
The performance of stress echocardiography in the diagnosis of ischemia is based on the concept of the «ischemic cascade»:160 In the presence of ischemia, in first place disturbances in myocardial perfusion occur, which can be detected by radionuclide scan and perfusion echocardiography. Then, disturbances in diastolic function appear, which can be visualized by pulsed transmitral Doppler or M-mode color Doppler and, regionally, by tissue Doppler and studies of strain (tissue deformation). Eventually, disturbances in systolic function that can be detected by stress echocardiography occur (Figures 10 and 11). The, finally, ECG disturbances and angina appear.
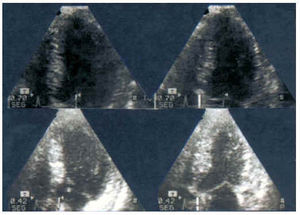
Fig. 10. Normal response in exercise stress echocardiography. In the apical 4-chamber plane, an overall improvement in the ejection fraction is visible, passing from 64% to 74%, with normal thickening and displacement of all segments. Above: baseline echo; below: peak exercise (150 bpm). Left: end-diastolic images; right: end-systolic images.
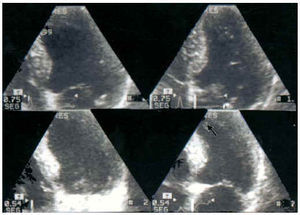
Fig. 11. Ischemic response of the region of the anterior descending coronary. In the apical 4-chamber plane, apical akinesia can be observed with exercise (arrow). Above: baseline echo; below: peak exercise (131 bpm). Left: end-diastolic images; right: end-systolic images.
Numerous studies have been published on exercise echocardiography on the treadmill and ergometric bicycle, reporting a sensitivity of 80% and a specificity of 85-90%. The diagnostic precision is always greater than that of conventional effort tests161-165 and generally close to that of radionuclide studies.161,166 In exercise echocardiography, regional and overall contractile function are analyzed at baseline and at peak effort167,168 or in the immediate postexercise period. Interpretable images are commonly obtained in more than 85% of patients. Limitations to the sensitivity of this technique are the need for special training, particularly when postexercise images are acquired, since there is a narrow «temporal window» for acquiring them, ideally <1.5 min. Other limitations are those of conventional exercise testing: inability to walk and failure to attain an adequate tachycardization.
Comparative studies of exercise, dobutamine, and dipyridamole have demonstrated a greater sensitivity (and similar specificity) of exercise and dobutamine than with dipyridamole,169,170 and scant serious undesirable effects.171 The addition of atropine to dobutamine increases the precision of the test and is especially useful in the presence of beta-blocker treatment.172 Transesophageal stress echocardiography has been carried out with dobutamine with considerable diagnostic safety and precision.173 Echocardiography with ergonovine can play a role in patients with suspected Printzmetal angina and negative results of other tests.174 Recently, excellent results have been communicated with transthoracic echocardiography obtained by oral introduction of a transesophageal electrical stimulation probe.175,176
Assessment of the coronary reserve
Coronary reserve represents the capacity of the coronary arteriolar bed to dilate in response to increased cardiac metabolic needs.177 It is calculated as the ratio between the velocity of coronary flow after the administration of a vasodilator (usually adenosine or dipyridamole) and in baseline circumstances. The advantages of adenosine are rapid action and termination of its effect and almost peak vasodilator capacity. Its disadvantage is more frequent side effects.178 Coronary reserve decreases progressively when a fixed lesion produces a stenosis of 50%, in such a way that it is completely suppressed with lesions of more than 90%. Its usefulness in the functional study of coronary lesions between 30% and 70% is unquestionable because it is in this range of angiographic severity that clinical doubts arise.
Transesophageal echography allows the visualization of the proximal segments of coronary arteries (generally the left main trunk and the first centimeters of the circumflex artery and anterior descending artery), but the latest generation equipment allows transthoracic visualization of flow through the distal portion of the anterior descending artery (Figure 12). This has the advantage of allowing the coronary reserve to be calculated at that level. Visualizing the flow of the anterior descending artery requires experience, as well as the use of modified planes. The possibility of measuring the distal coronary reserve is excellent because the reserve calculated reflects the residual vasodilator capacity of the vascular bed, which is affected specifically by proximal lesions of the proximal and middle anterior descending coronary.179-181 Caiati et al,182 who studied the coronary reserve with the infusion of adenosine using pulsed Doppler in the distal section of the anterior descending artery, reported a sensitivity of 91% and specificity of 76% for detecting stenosis >75%. The flow signal was optimal without contrast in half of the patients (55%) and in all of them after contrast was administered. However, it must be considered that the coronary reserve is also reduced in left ventricular hypertrophy,183 small-vessel disease, constrictive pericarditis,184 cardiomyopathies,185 transplanted hearts,186 and left bundle-branch block.187
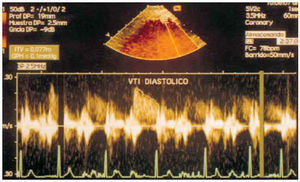
Fig. 12. Diastolic flow of the distal anterior descending coronary artery obtained by transthoracic pulsed Doppler in baseline circumstances.
Echocardiography of myocardial perfusion
Myocardial perfusion echocardiography is the technique that, in combination with vasodilators, results in the earliest detection of ischemia by revealing a heterogeneous flow, as radionuclide techniques do. The main difference with respect to myocardial radionuclide perfusion scan is that the thallium or technetium compounds are deposited in the cardiac cell, indicating cellular integrity or viability, whereas perfusion echocardiography only indicates the integrity of the microcirculation.
At present, contrast agents are available that can be used intravenously thanks to special characteristics that allow them to pass the pulmonary barrier without being destroyed. Nevertheless, it still has not been established which is the best method for detecting echocontrast in the myocardium. The contrast agents produce a significant amount of bubbles that enter the coronary circulation and expand and compress alternatively when exposed to ultrasound. The detection of these bubbles depends on their capacity to generate two resonance waves of different frequency, in contrast with myocardial tissue. Therefore, if ultrasounds are emitted to the myocardium at a frequency of 1.8 mHz, the myocardium itself will not transmit any frequency at 3.6 mHz, but the bubbles will. Therefore, by changing the system reception frequency to 3.6 mHz, the echoes from the bubbles will be visualized without the echoes from the myocardium. The second point to consider is that ultrasound rapidly destroys the bubbles, especially if the so-called «mechanical index» is high. The intermittent imaging system is the system of transmitting and receiving ultrasounds only once every x cycles. The bubbles can be destroyed by the fleeting emission of ultrasounds, but in the meantime new bubbles that can be detected enter the system.
At present, numerous problems still exist, due mainly to artifacts. To determine if the echoes originating from the myocardium represent true perfusion or artifacts, the image can be multiplied, as well as intermittent. Multiplication consists of the emission and reception of ultrasound by the system at a moment in the cardiac cycle (image 1), and immediately after, in 30-50 ms (image 2). If «perfusion» is visible in image 1 and not in image 2, it is probably true perfusion, because the bubbles in image 1 will have been destroyed and the time is too short for the myocardium to have filled again. If perfusion is appreciated in both images, it usually should be considered an artifact. Another system for differentiating between true perfusion and artifacts is to change the frequency of the multiple images, passing from emitting and receiving, for example, every 4-5 beats in which perfusion is observed, to emitting and receiving every beat, in which it should not be visible due to the destruction of the bubbles.
Studies made with intermittent and multiple images using dipyridamole188 or adenosine,189 and comparing this technique with SPECT and technetium compounds, have obtained similar results. Agreement between methods for the presence or absence of coronary artery disease in each patient was 81-86%. Nevertheless, the agreement between techniques was greater in the territory of the anterior descending artery (81%; K=0.81) and right coronary (76%; K=0.52), and significantly smaller in the territory of the circumflex artery (72%; K=0.40). This is due to the large number of false defects obtained with echocontrast on the lateral face. In effect, due to the angle of incidence of ultrasound on the lateral wall, false defects may be more common in this territory. Spencer et al 190 also demonstrated a greater frequency of perfusion defects with echocontrast in the lateral wall, in the absence of defects in thallium radionuclide scans, with both techniques carried out at rest.
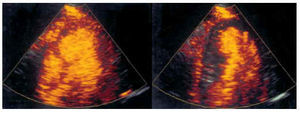
In addition to the system for detecting perfusion by intermittent and multiple images, bubbles can currently be visualized in real-time images. The aim is to work with a very low mechanical index that does not destroy the bubbles. To check if true perfusion is involved, at a given moment an emission with a high mechanical index (flash) will destroy the bubbles present in a true perfusion (Figure 13). The initial results obtained with these techniques seem promising, because they overcome many of the limitations of perfusion echocardiography,191,192 allowing them to be used, for example, during stress tests.193,194
Fig. 13. Four-chamber image of a real-time echocontrast perfusion study (mechanical index 0.1) in a patient with extensive anterior akinesia after anterior infarction. On the left can be observed signals due to echocontrast in the entire myocardium. On the right is seen the destruction of intramyocardial bubbles when the mechanical index is suddenly increased to 1.4.
Possible, or future, clinical applications of myocardial perfusion echocardiography are not only the detection of ischemia, but also the detection of viability. Studies that have compared vascular integrity by echocontrast with the inotropic reserve by echodobutamine to predict recovery in coronary patients with ventricular dysfunction have documented a good sensitivity with the echocontrast (80-95%), but a notably lower specificity than with dobutamine echocardiography (50-75% vs 85%).195-199 Myocardial perfusion echocardiography can also evaluate the non-reflux phenomenon. It is known that approximately 18-25% of patients have impaired microcirculation after an effective primary angioplasty or fibrinolysis (TIMI-3), and that these patients do not recover their microcirculatory function during follow-up.200,201
CONCLUSIONS
The intracoronary hemodynamic parameters that reflect the physiology of coronary circulation correlate acceptably with the functional assessment of myocardial ischemia evaluated by myocardial perfusion SPECT and stress echocardiography. However, none of these techniques allows absolute quantification of coronary flow and discrepancies can be observed between them in up to 15% of patients.
From the review of more than 7000 patients included in 75 studies in which SPECT and either stress or pharmacological stress echography110 were available, it was concluded that effort SPECT was more sensitive than effort echography for the diagnosis of coronary artery disease, identification of stenotic arteries, and diagnosis of multivessel disease. In a review of 1380 patients from 22 studies in which there was a direct comparison of SPECT and stress echography (effort, dobutamine, adenosine, or dipyridamole),111 SPECT was found to be more sensitive but less specific than echography, particularly in the identification and localization of nonsevere coronary artery disease and if vasodilators were used as the provocation maneuver. The greater tradition of radionuclide scans has probably contributed to its selection as the reference method for invasive functional studies,42-45,47-49,202 but in practice the indication for each examination depends mainly on the availability and experience with each technique in individual hospitals. The most desirable aim is that each center control its own results insofar as the diagnostic and prognostic effectiveness of the method that it uses. In this sense, different studies have shown that complications in patients with ischemic heart disease correlate more with the amount of ischemic myocardium revealed by effort or pharmacological stress than with the number of angiographically stenotic arteries.203-208 Therefore, in the future decisions about coronary revascularization must also be based on evidence that a certain coronary stenosis also has objective functional significance.
Correspondencia: Dr. J. Candell-Riera.
Servicio de Cardiología. Hospital Universitari Vall d'Hebron.
P.° de la Vall d'Hebron, 119-129. 08035 Barcelona. Spain
E-mail: jcandell@hg.vhebron.es