Keywords
INTRODUCTION
Numerous studies in young patients have shown a relationship between cryptogenic stroke and isolated patent foramen ovale (PFO) or PFO with concomitant atrial septal aneurysm (ASA).1-4 However, until recently, only few studies have addressed this association in older patients,2,5-7 and the results were contradictory. Two recent studies have shown an association between atrial septal defects and cryptogenic stroke in older patients. However, in one of these studies,8 some of the patients may not have had cryptogenic stroke, since transesophageal echocardiography (TEE) was not used systematically to rule out other causes of stroke, such as atherosclerotic disease of the aortic arch.9-11 In the other study,12 isolated PFO was no more frequent in the group of older patients with cryptogenic stroke than in those with stroke of known cause, whereas PFO with concomitant ASA was more common in the group with cryptogenic stroke.
In this study, we used TEE to assess the prevalence of PFO in patients with cryptogenic stroke and compared the results obtained in patients aged at least 55 years with those in patients younger than 55 years.
METHODS
A prospective study was performed of all patients admitted to the neurology department with a presumptive diagnosis of cryptogenic stroke between January 2000 and May 2008. Patients were older than 14 years with no identifiable cause of stroke following a complete examination. Stroke was defined as cerebrovascular accident (rapid-onset focal neurologic deficit that persists fully or partially for more than 24 hours in surviving patients) or transient ischemic attack (rapid-onset focal neurologic deficit that is fully resolved in the first 24 hours). Stroke etiology was classified according to the criteria recommended by the Working Group on Cerebrovascular Disease of the Spanish Society of Neurology,13 which are similar to the TOAST classification14 and include 5 etiologic types based on clinical findings and the results of diagnostic tests. One of the types is stroke of unknown or undetermined cause.
The study protocol included the following elements:
- Systematic general examination. In all patients a detailed clinical history was taken along with a complete assessment of etiology comprising chest x-ray, electrocardiogram, Holter monitoring in the case of suspected supraventricular arrhythmia, cerebral imaging (computed tomography or magnetic resonance imaging), examination of the supra-aortic branches and cerebral arteries (Doppler study of the supra-aortic branches and/or computed tomography angiography and/or cerebral magnetic resonance angiography), and blood workup to rule out hypercoagulability.
- Cardiology examination. This included transthoracic echocardiography (TTE), which was performed whenever there was suspicion of cardioembolic stroke or in the absence of other causes identified in the prior etiologic study. When the result was normal or inconclusive, TEE was requested with assessment of PFO and aortic atheromatosis. Echocardiography was performed with an Acuson®
Sequoia ultrasound system with a multifrequency second harmonic transthoracic probe (2.5-5 MHz) and a multiplane, multifrequency transesophageal probe (3.5-7 MHz).
TEE was used in all cases to assess the etiology of stroke with special attention to the anatomy of the atrial septum, assessment of PFO, examination of the aorta and possible aortic atheroma, small tumors or excrescences on valves and septa, spontaneous echo contrast, and thrombi in the atrium or left atrial appendage.
The anatomy of the atrial septum was assessed and ASA was diagnosed when the total excursion of the septum into the left atrium, right atrium, or the sum of the excursion into both atria was at least 11 mm.15
For assessment of PFO the brachial vein was injected with 4% gelafundin in sterile saline. In all cases, injections were performed at rest and following Valsalva maneuver. PFO was diagnosed when 3 or more microbubbles were seen to pass to the left atrium within the first 3 beats of their arrival at the right atrium, both at rest and following Valsalva maneuver. An echocardiography specialist quantified the number of microbubbles passing from right to left atria; PFO was diagnosed as mild when the number of microbubbles in a cycle was <20 and extensive when 20 or more microbubbles were observed in a cycle figure 1 the size of pfo was also assessed at rest by measurement maximum aperture between septum primum secundum vertical plane p
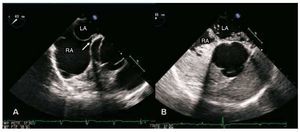
Figure 1. A: visualization of the foramen ovale in the form of a tunnel (arrow) by transesophageal echocardiography (retroaortic projection). B: extensive passage of contrast material (sterile gelfundin) through the patent foramen ovale, in the same projection. LA indicates left atrium; RA, right atrium
The ascending aorta and aortic arch up to the left subclavian artery were examined, and 3 grades of atheromatosis were classified according to the modified criteria of the French Study of Aortic Plaques in Stroke Group10 (grade I, plaques <4 mm grade ii plaques 8805 4 iii of any thickness with an addition mobile intraluminal component the thickest plaque was used for classification and or were considered complex
Cryptogenic stroke was diagnosed when the study failed to identify any cause reported in the literature to be associated with stroke, including aortic atheromatosis with complex grade II or III plaques.
Study Groups
A total of 262 consecutive patients with stroke in whom no cause had been identified in any of the diagnostic tests, including TTE, were referred for study with TEE. All were in sinus rhythm. Patients were classified in 3 groups according to age and ultrasound findings:
- Group A: patients aged ≥55 years with cryptogenic stroke. These patients formed the main study group.
- Group B: patients aged <55 years with cryptogenic stroke p
- Group C: patients aged ≥55 years in whom complex aortic atheromatosis (grade II and III) was observed by TEE. These patients were diagnosed as having atheroembolic stroke and represented a control group for Group A.
Exclusion criteria
Patients were excluded from groups A and B if other recognized causes of stroke were identified in the TEE.
The occurrence of PFO and atrial septal aneurysm was studied in the 3 groups and data from Group A (study group) were compared with those from Group B and Group C (control).
Statistical Analysis
The statistical analysis was carried out using the SPSS statistical package, version 12 for Windows. Continuous variables were expressed as means (SD). Categorical variables were expressed as percentages. The Kolmogorov-Smirnov test was used to assess whether variables obeyed a normal distribution. Quantitative variables were compared by t test for normally distributed variables and Mann-Whitney U test for variables with a non-normal distribution. Between-groups comparisons of qualitative variables were done with the Pearson χ2 test or the Fisher exact test. Values of P<.05 were considered to be statistically significant.
RESULTS
Of the 262 patients referred for TEE, 68 were aged at least 55 years. In 24 (35%) of those patients, stroke was not considered cryptogenic due to identification of complex atheroma plaques in the aortic arch by TEE (Group C), whereas in the remaining 44 (65%) patients it was considered to be cryptogenic stroke (Group A) on the basis that no other cause of stroke was identified. The other 194 patients were aged less than 55 years, and all were diagnosed with cryptogenic stroke (Group B). General clinical characteristics are shown in Table 1, which compares the 2 groups of patients with cryptogenic stroke (Groups A and B), and in Table 2, which compares the 2 groups of older patients (Groups A and C).
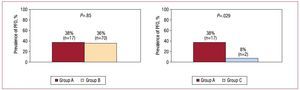
In both groups of patients with cryptogenic stroke, there was a high frequency of PFO, with no significant differences between age groups (38% in Group A vs 36% in Group B; P>.05) (Figure 2). However, PFO was more common in Group A than in Group C (38% vs 8%; P=.029) (Figure 2). There were also no differences in the size of PFO between groups A and B (2.1 [2.5] mm vs 4 [1.7] mm; P>.05) or in the transit of contrast agent, which was extensive in most cases (87% in Group A vs 77% in Group B; P>.05). There were no cases of significant passage of contrast agent through a PFO in Group C (P<.01 compared with Group A).
Figure 2. Prevalence of patent foramen ovale (PFO) in the 3 study groups and comparison of the main study group (Group A) with the other 2.
Similar results were obtained for PFO with concomitant ASA. There was no significant difference in frequency between the groups of patients with cryptogenic stroke (18% in Group A vs 11% in Group B; P>.05) (Figure 3) but there was a higher frequency in Group A than Group C (18% vs 0%; P=.039) (Figure 3). However, the presence of aneurysms not associated with PFO was more common in Group A than Group B (4.8% vs 0.5%; P<.05) and slightly lower than in Group C (4.8% vs 8%), although the difference did not reach statistical significance.
Figure 3. Prevalence of patent foramen ovale (PFO) associated with atrial septal aneurysm (ASA) in the 3 study groups and comparison of the main study group (Group A) with the other 2.
Among the clinical characteristics of stroke, there was a higher percentage of acute cerebrovascular accidents in the older patients in groups A and C (Table 2), whereas transient ischemic attack was more common in patients from Group B (Table 1).
The frequency of cardiovascular risk factors was similar in both groups of older patients (Table 2). In contrast, diabetes and hypertension were less frequent in patients from Group B than in those from Group A, whereas smoking was more common in Group B (Table 1).
Clinical signs or symptoms of deep vein thrombosis were only present in 3 patients from Group A (associated in 1 case with pulmonary thromboembolism) and 2 patients from Group B, all of whom had PFO. There were no cases in Group C.
DISCUSSION
Cryptogenic stroke is less common in older patients than in their younger counterparts.2,8 In our experience, covering almost 8 years of exhaustive study, we have only encountered 44 cases of cryptogenic stroke in older patients, compared with 194 cases in younger patients. However, cryptogenic stroke in older patients represents a major health care challenge, since minimally invasive diagnostic techniques such as TEE are underutilized and yet could reveal new etiologies for this disease.
When studied systematically with TEE in all patients with stroke of unidentified cause, older patients with cryptogenic stroke have a similar prevalence of PFO to that seen in younger patients, and a significantly higher prevalence than seen in older patients with cardioembolic stroke. Although this association between PFO and cryptogenic stroke has been clearly demonstrated in the literature for younger patients,1-4,16,17 the data have been less clear for older patients. Only a small number of case-control studies have been undertaken in older patients and the results were contradictory, largely due to the use of different techniques for the diagnosis of PFO.5-7 As a result, meta-analyses such as that reported by Overell et al2 could not provide conclusive data.
The study by Handke et al8 also found a higher prevalence of PFO in older patients with cryptogenic stroke than in those with stroke of known cause. However, the prevalence of PFO in older patients with cryptogenic stroke was lower than in younger patients (28% vs 44%), probably because many cases were not authentic cryptogenic stroke, as a result of having failed to study the intracranial vessels in half of the cases. Unlike in our study and others,18 these authors did not use TEE systematically, and as a result, not all of the patients would have authenticate cryptogenic stroke. In our study, this was the case in 35% of older patients, who proved to have complex atheromatous plaques in the aortic arch according to the result of TEE.
A recent study involving systematic use of TEE for the classification of stroke only observed a trend towards statistical significance for the increased frequency of PFO in older patients with cryptogenic stroke.12 However, that study was limited by having a retrospective design.
Similar results to those seen for isolated PFO were obtained for PFO with concomitant ASA, with a higher prevalence in both groups of patients with cryptogenic stroke than in older patients with stroke of known cause. These results are consistent with the results obtained in more recent studies involving older patients with cryptogenic stroke.8,12 Mas et al19 reported that the occurrence of PFO and ASA was associated with an increased risk of stroke due to paradoxical embolism, a finding that contrasts with the low prevalence (1.7%) of this association in the general population.20 Thus, our results may indicate that the presence of PFO and ASA might also be associated with an increased risk of stroke in older patients. Nevertheless, the frequency of isolated ASA without PFO in the 2 groups of older patients with stroke was notably lower than the association of PFO and ASA, with no differences between the groups, a finding that is consistent with previous reports.12
TEE offers the advantage of providing anatomical and functional data relating to PFO, such as the extent of right-to-left shunt. This was extensive in most cases of cryptogenic stroke in both age groups, a finding that is consistent with previous reports describing the relationship between the extent of passage through the PFO and the risk of stroke.16,17 Something similar occurs with the size of the PFO,16,17which was also greater in both groups of patients with cryptogenic stroke, although this difference did not reach statistical significance, probably as a result of the low frequency of PFO in older patients with atheroembolic stroke.
As mentioned, the advantage of our study over others carried out in patients with cryptogenic stroke is that TEE was used systematically. Although TEE has increased the diagnostic potential during assessment of cryptogenic stroke, its indications for cerebral ischemia are not well established,21 and its use is restricted in older patients. However, our results suggest that TEE should be systematically included in the assessment of the cause of cryptogenic stroke in this age group, since it allows other potentially treatable causes to be detected. In our study, this was the case in 35% of older patients with complex atheromatous plaques in the aortic arch, a frequency that is very similar to that reported in other studies.9
TEE offers advantages over other diagnostic techniques for the study of PFO, such as TTE, which has a lower sensitivity22 and poorer definition of the anatomy of the atrial septum. In contrast, transcranial Doppler has a high sensitivity for the detection of right-to-left shunt, but does not discriminate between intracardiac and extracardiac shunt and provides no information on the anatomy of PFO.23
In terms of the clinical characteristics of the patients in this study, the distribution of cardiovascular risk factors between the 3 groups was similar to that described in the literature,24,25 with no differences between the 2 groups of older patients and a lower prevalence in younger patients with cryptogenic stroke, except for smoking.
Few cases had evidence of deep vein thrombosis, consistent with other studies addressing PFO and cryptogenic stroke.26,27 Furthermore, systematic assessment of deep vein thrombosis was of limited diagnostic value in those studies. Nevertheless, pathophysiologic findings that are common to this age group, such as the increased likelihood of paradoxical embolism28 and venous thromboembolic disease,29 alongside the increased frequency of PFO in older patients with cryptogenic stroke, as in our study, appear to support a causal relationship between PFO and stroke in patients 55 years of age or older.
The presence of PFO in older patients with cryptogenic stroke is associated with an increased risk of adverse events, despite antiplatelet or anticoagulant therapy, whereas this is not the case in younger patients.30 This suggests that older patients with cryptogenic stroke should be studied in greater depth and treated more aggressively. Currently, there is no evidence supporting the superiority of percutaneous closure over medical treatment in patients with cryptogenic stroke and PFO,31 and although ongoing randomized trials (RESPECT, CLOSURE) may provide some clarification, they have enrolled almost no patients aged over 60 years, and these are the patients who could most benefit from this treatment strategy.
Limitations
No systematic TEE study was performed in patients aged at least 55 years in whom a cause of stroke was identified in the general tests, since the test was carried out at the request of the neurologist, and as a result it was not possible to assess the prevalence of PFO in this group.
Since the study was undertaken in patients as part of daily clinical practice, there was not control group of older patients without cerebrovascular disease.
Despite an enrolment period of almost 8 years, the main study group (patients ≥55 years) was not very large, and this may have limited the statistical power of some of the results. However, we believe that this reflects the very low prevalence of cryptogenic stroke in older patients when an extensive study is undertaken to identify the cause of stroke. The control group of older patients with severe atheromatosis observed by TEE was also quite small, since the cause of stroke was identified in most patients as part of the routine study, a finding that is also consistent with the percentages reported in the literature.
Finally, patients in whom other causes of stroke were observed in TEE were excluded, with the exception of those older patients with complex atheromatous plaques in the aortic arch, who formed the control group. As a result, other uses of TEE in this context cannot be assessed as a result of having focused on PFO.
CONCLUSIONS
Patients of all ages (older or younger than 55 years) with cryptogenic stroke have a higher frequency of PFO, with or without ASA, compared with patients with similar characteristics but with cardioembolic stroke, as indicated by the presence of complex aortic atherosclerotic plaques. This indicates that paradoxical embolism could be the underlying pathophysiologic cause of this type of stroke.
In older patients with stroke of unknown cause, TEE offers a high diagnostic yield for the detection of complex atheromatous plaques in the aortic arch as a cause of atheroembolic stroke. Although this group represents a minority of older patients with ischemic stroke, systematic study with TEE in these patients could have important therapeutic implications.
ABBREVIATIONS
ASA: atrial septal aneurysm
PFO: patent foramen ovale
TEE: transesophageal echocardiography
TTE: transthoracic echocardiography
Correspondence: Dra. D. Mesa Rubio.
Ctra. de las Ermitas, 65. 14012 Córdoba. Spain.
E-mail: dmesar@ya.com
Received May 30, 2009.
Accepted for publication November 25, 2009.