Keywords
INTRODUCTION
Aldosterone, the main mineralocorticoid synthesized by the adrenal gland, has an essential function in sodium and water homeostasis and urinary excretion of potassium. Nonetheless, it has become evident in recent years that this hormone also has an important pathogenic role in hypertension and vascular remodeling, in left ventricular hypertrophy, and in renal disease, specifically proteinuria and glomerulosclerosis in patients with hypertension.1 A clear example of this association is primary hyperaldosteronism, a secondary cause of hypertension (HT), in which an increase in plasma aldosterone concentration produces a significant deleterious effect on the heart and vessels and leads to a higher risk of experiencing cardiovascular events.2
In advanced phases of renal failure, aldosterone values increase significantly as glomerular filtration decreases, due to activation of the renin-angiotensinaldosterone system secondary to the change in glomerular hemodynamics. This contributes to greater organ damage, affecting both the kidneys and heart.3,4 In hypertensive patients with preserved renal function, however, there are no studies specifically investigating the association between aldosterone concentration and renal filtration. The aim of this study was to analyze the relationship between plasma aldosterone concentration and the glomerular filtration rate (GFR) in hypertensive patients with preserved renal function, after ruling out hyperaldosteronism.
METHODS
During the period of 2005 to 2008, a prospective study was conducted in all patients with hypertension older than 18 years of age referred to the hypertension unit of our cardiology department. A clinical history and complete physical examination were performed in all patients, together with specific laboratory tests to rule out a secondary cause of hypertension (HT). Samples for laboratory testing were taken after a 12-hour fast. The tests included a hemogram and routine biochemical analyses, thyroid hormones, aldosterone, the plasma aldosterone-to-plasma renin activity ratio (ALD/PRA), urinary catecholamines and cortisol, and microalbuminuria in 24-hour urine. Renal function was estimated by calculating the GFR with the Modification of Diet in Renal Disease (MDRD) formula. Renal dysfunction was established on GFR values of <60 mL/min. Left ventricular mass was determined by echocardiography using the Penn formula.
Patients were excluded if they had a diagnosis of primary hyperaldosteronism as defined by an ALD/PRA ratio >30, together with elevated plasma aldosterone (>20 ng/dL) and a lack of plasma aldosterone suppression following saline load during 4 hours. Patients with decreased renal function (GFR <60 mL/min) were also excluded. Patients were divided into 2 groups according to the GFR (60-89 mL/min and >90 mL/min), based on the chronic renal disease stages established by the National Kidney Foundation (stages 1 and 2).
Statistical Analysis
Continuous variables are expressed as the mean (standard deviation), and qualitative variables as the number and percentage. For the analysis of correlations between the quantitative variables, ALD and GFR, the Spearman correlation test was used, since the variables did not follow a normal distribution. A multiple linear regression analysis was then performed, adjusting for age, sex, diabetes mellitus, smoking habit, dyslipidemia, prior ischemic heart disease, evolution time of HT, systolic and diastolic arterial pressure, left ventricular mass adjusted by body surface area and determined by echocardiography, and plasma aldosterone concentrations. Because aldosterone concentrations did not follow a normal distribution, logarithmic transformation of this variable was carried out. The statistical analysis was performed with SPSS, version 13, and differences were considered significant at a P value of <0.05.
RESULTS
After excluding patients with hyperaldosteronism and/or GFR <60 mL/min, 186 patients with hypertension were included (58.1%, men), with a mean age of 55 (13.4) years. The main clinical characteristics of the population are presented in Table.
Of the patients enrolled, 77 had a GFR of >90 mL/min and 109 had a GFR of 60 to 89 mL/ min. Patients with GFR 60 to 89 mL/min showed significantly higher aldosterone values than the group with GFR >90 mL/min (20.02 vs 15.3 ng/ dL; P<.05). More patients in the group with lower GFR had a history of ischemic heart disease, left ventricular hypertrophy on echocardiographic study, and elevated plasma N-terminal probrain natriuretic peptide (NT-proBNP) concentrations (Table). There were no differences between the 2 groups in the pharmacological treatment received, except for a higher use of statins in patients with a GFR of 60 to 90 mL/min that approached significance (32.1% vs 19.5%; P=.06).
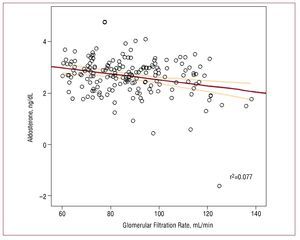
There was a modest negative correlation between plasma aldosterone concentrations and GFR (r=-0.196; P<.01), in which higher serum aldosterone values were associated with lower glomerular filtration (Figure). After adjusting for several variables, the multivariate analysis confirmed an independent association between serum aldosterone concentrations and the GFR in patients with hypertension (B=-7.36; P<.001). The other associated variables included age (B=-0.58; P<.001) and systolic arterial pressure (B=-0.13; P<.05).
Figure 1. Spearman rho correlations between plasma aldosterone concentration and glomerular filtration rate.
DISCUSSION
The present study yielded an interesting finding that has not been analyzed in depth previously: an independent association between plasma aldosterone concentrations and renal filtration in hypertensive patients with "normal" renal function, such that the lower the renal filtration values, the higher the plasma aldosterone concentration. Although the correlation was modest, this is the first study describing this association in the incipient phases of hypertensive renal damage.
In the last years, considerable attention has been focussed on the contribution of aldosterone to the pathophysiology of HT and cardiovascular disease. Various studies have shown that in comparison to patients with essential HT, patients with primary hyperaldosteronism and elevated aldosterone values have higher rates of albuminuria and worsening renal function5 as well as a larger number of cardiovascular events and a greater degree of left ventricular hypertrophy and vascular remodeling.6
In addition, the results from several clinical trials support the hypothesis that aldosterone can independently produce renal and cardiac damage in patients without hyperaldosteronism.7 It is known that in advanced stages of renal failure, aldosterone values usually increase secondary to activation of the renin-angiotensin-aldosterone system, and this can contribute to greater organ damage and create a vicious circle.3,4 Nonetheless, in hypertensive patients with preserved renal function, there are no clinical reports that specifically investigate the possible relationship between aldosterone and renal function.
It is mainly in experimental animal studies where the damage caused by aldosterone has been analyzed at different levels of the nephron, such as the mesangium, basement membrane, and renal tubule. These studies indicate an important pathologic role of the hormone in renal function deterioration.8-10 It seems clear that aldosterone is an important mediator of collagen turnover, stimulating the expression of various profibrotic molecules and inhibiting other antifibrotic molecules, thereby assuming a decisive role in the development of renal and cardiac fibrosis.11 In this last aspect, the present study brings to light a possible deleterious effect of aldosterone on the heart, as manifested by a higher prevalence of ischemic heart disease in patients with higher aldosterone concentrations, which has been described previously in patients with hyperaldosteronism,6 and a significant correlation with the left ventricular mass adjusted by body surface area (r=0.167; P<.05).
Our findings underscore the interesting association between plasma aldosterone concentration and deteriorated renal function in the initial phases of patients with hypertension. Studies in this field are needed to analyze whether more effective and intensive blockade of aldosterone secretion could be effective in preventing renal function deterioration in these patients.
Correspondence: Dr. P. Morillas Blasco.
Servicio de Cardiología. Hospital Universitario San Juan. 03550 San Juan de Alicante. Alicante. España.
E-mail: pedromorillas@teleline.es
Received December 20, 2008.
Accepted for publication May 12, 2009.