Mechanical circulatory support with a left ventricular assist device (LVAD) is an established treatment for patients with advanced heart failure.1,2 Nonetheless, it is not free from complications, with right ventricular failure (RVF) being one of the most dreaded postprocedure events.3,4 Despite adequate risk stratification and optimal periprocedure treatment, some patients experience RVF and require a second circulatory assist device.3 Several devices and implantation techniques have been used for this purpose.5 We present the case of a patient who was treated with LVAD implantation, developed RVF, and underwent placement of a device for percutaneous venous-to-pulmonary artery extracorporeal membrane oxygenation (ECMO) for percutaneous right ventricular support.
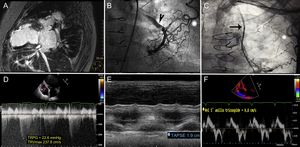
A 64-year-old woman with a history of dilated ischemic cardiomyopathy had severely depressed left ventricular function. In 2002, she underwent coronary artery revascularization surgery for disease of the left anterior descending artery and first diagonal artery. A left mammary to left anterior descending artery bypass and a saphenous to diagonal artery bridge were performed. The patient did not continue follow-up after the procedure. In 2015, she was admitted for heart failure. During hospitalization she showed severe left ventricular dysfunction (left ventricular ejection fraction 21%, end-diastolic volume 87mL/m2, end-systolic volume, 37mL/m2), and magnetic resonance imaging confirmed an absence of viability in the territory of the left anterior descending artery (Figure 1A). Coronary angiography showed a complex fistula that connected the left mammary arteries and left subclavian artery with the left inferior lobar artery (Figure 1B and C). The vascular malformation was percutaneously closed with coils, but the patient had a poor clinical course with rehospitalizations for heart failure, dependence on inotropic treatment, and an INTERMACS profile of 3. She was evaluated for heart transplantation, but was rejected because of major peripheral vascular disease (Leriche syndrome). Therefore, LVAD implantation was decided as destination therapy. Her previous study showed normal right ventricular (RV) function (Figure 1 D-F and video 1 of the supplementary material), and the following values were recorded on right heart catheterization: right atrial pressure, 7mmHg; mean pulmonary artery pressure, 25mmHg; pulmonary artery wedge pressure, 20mmHg; cardiac index, 1.5 L/min/m2; and pulmonary vascular resistance, 2.5 WU. The right atrial pressure/pulmonary artery wedge pressure ratio was 0.35 and the RV stroke work index was 415mmHg/mL/m2.
A. Cardiac magnetic resonance imaging; transmural delayed enhancement in the anterior territory. B and C. Fluoroscopy images; arteriovenous fistula (asterisks), left mammary artery (arrow), lobar artery (arrowhead). D. Echocardiogram; continuous Doppler of mild tricuspid insufficiency. E and F. M-mode and tissue Doppler; normal left ventricular function. TAPSE, tricuspid annular plane systolic excursion; *TRPG, peak tricuspid regurgitation pressure gradient; TRVmax, tricuspid regurgitation maximum velocity.
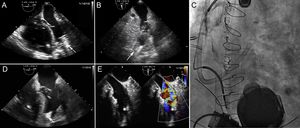
A HeartMate 3 device (Abbott) was implanted through a conventional sternotomy. The patient underwent the standard preimplantation protocol used in our center: inodilator treatment with levosimendan 24hours before and nitric oxide during the procedure. Even though she had no previous risk of RVF, she showed data indicative of this complication in the immediate postoperative period. She required vasoactive support with adrenaline and milrinone, and was dependent on nitric oxide (Figure 2A and B). Despite these measures, the RVF progressed and a decision was made to implant a device (CardioHelp system, Maquet Cardiopulmonary AG) for percutaneous venous-pulmonary artery ECMO. The procedure was carried out under radiologic/echocardiographic guidance. A 21-Fr drainage cannula was implanted through the right femoral vein with the distal portion lodged in the right atrium. To place the return cannula, a Berman pressure-rated catheter was implanted through the right jugular vein and a high-support 0.035-inch Amplatz Super Stiff guidewire was advanced through the catheter. This support enabled direct implantation of a Bio-Medicus 15-Fr × 50-mm pulmonary artery cannula (Medtronic). The cannula's flexible design averts injury to the RV during implantation. The distal portion was lodged in the main pulmonary artery before the bifurcation (Figure 2C and video 2 of the supplementary material).
A. TEE in 4-chamber view; severe RV dilatation with LV collapse and suction by the LVAD. B. TEE in 2-chamber view; LV collapse. C. Fluoroscopy image; LVAD and venous-pulmonary artery ECMO in normal position. D. TEE in 4-chamber view; less RV dilatation and improved LV collapse. E. TEE of the distal ECMO cannula in the pulmonary artery (On the images, LVAD drainage cannula indicated by an arrow, distal cannula of ECMO indicated by an asterisk). ECMO, extracorporeal membrane oxygenation; LV, left ventricle; LVAD, left ventricular assist device; RV, right ventricle; TEE, transesophageal echocardiography.
Immediately after initiating ECMO support, filling improved in the left chambers and there was an increase in the LVAD output (Figure 2D and E, and video 3 of the supplementary material). The inotropic support was decreased and right chamber function improved, enabling withdrawal of ECMO by manual compression after 3 days of assistance. Unfortunately, the patient died 1 week later due to sepsis.
Despite advances in perioperative treatment, an estimated 6% of patients who receive an LVAD experience RVF requiring mechanical assistance.3 There are several options for RV circulatory support, but most are adaptations of devices designed for left ventricular assistance (eg, Thoratec CentriMag, and Maquet Rotaflow), which require surgical implantation and are not free from complications.3,5 The development of devices specifically designed for the RV, such as the Impella-RP (Abiomed), and dual-lumen venous cannulas, such as the Protek-Duo (CardiacAssist), may offer benefits for these patients, but data on their performance are limited.6 ECMO with this type of cannulation may be a feasible option for patients with RVF of different etiologies, as it enables circulatory/respiratory assistance and reduces the complications associated with surgery.