Keywords
INTRODUCTION
Amplatzer devices are used in the percutaneous closure of ostium secundum atrial septal defects (OS-ASD),1 patent ductus arteriosus (PDA),2 and muscular ventricular septal defects (m-VSD).3 They are safe and entail little risk and this has led to their widespread use in adult and pediatric patients, but little is known of their use, effectiveness and safety in patients aged <1 year.4,5
METHODS
We conducted a retrospective review of children aged <1 year diagnosed with OS-ASD, PDA, or m-VSD, who underwent percutaneous closure procedures in our hospital between January 2001 and January 2008. We did not treat children with perimembranous VSD because these devices entail a risk of complete atrioventricular block, as recently confirmed for ≤5% of patients.6
For each defect type, we used the techniques described in the literature for older children and adults.1-3,7 In all patients, we used general anesthesia; in patients with OS-ASD or m-VSD we also used transesophageal echocardiography.
The Fick method was used to calculate the Qp/ Qs. We chose an appropriate device according to the defect diameter; in patients with OS-ASD, a sizing ballon was used to establish the stretched diameter of the OS-ASD, in ductus arteriosus, we measured at the pulmonary end in lateral aortic angiography; in m-VSD, diameter was estimated using echocardiography and/or the ventriculography in the left oblique projection. We employed nitinol-filament, self-expanding Amplatzer devices (AGA Medical Corporation, Golden Valley, Minnesota, USA) that have thermal memory and can be deployed in a controlled manner.
We recorded demographic, clinical and hemodynamic data, procedure duration recorded as radioscopy time, results, and immediate and late complications. Data are expressed as mean (range).
RESULTS
We treated 22 children aged <1 year with symptoms of OS-ASD, PDA or m-VSD (Table 1). Clinical indications, hemodynamic variables (Qp/Qs and AP/AO systolic pressure ratio), defect diameter and devices employed are in Table 2.
Radioscopy times for OS-ASD, PDA and m-VSD were 23.7 (13-40), 21.5 (6-60), and 44 (34-57) min, respectively.
All procedures were successful. We had no acute or late complications during follow-up of 18.7 (5.5-38.3), 34 (5.7-90.9), and 44.1 (20-65.2) months for OS-ASD, PDA, or m-VSD respectively.
We observed no peripheral vascular complications attributable to surgery or administration of heparin and/or thrombolytic agents.
DISCUSSION
Atrial Septal Defects
Most children with OS-ASD have few symptoms and surgery is generally indicated at pre-school age,8 although some require earlier treatment. It is reported that OS-ASD tend to grow with time.9
The group of children susceptible to early treatment includes those at greatest risk of progressing to irreversible pulmonary hypertension.10 For example, patient 1, with Down's syndrome, presented severe pulmonary hypertension with AP/AO ratio=1. His pulmonary pressure was normal at 1 year post-procedure. In patient 2, repeated respiratory infections, failure to thrive or high Qp/Qs indicated the treatment. Patient 3 (Figure 1), with congenital complete atrioventricular block without indication for pacemaker, had moderate pulmonary hypertension with AP/AO systolic pressure ratio =0.73 during cardiac catheterization. At 6 months, echocardiography showed normal pulmonary pressure. These 3 patients had specific indications for OS-ASD closure and we opted for percutaneous treatment to avoid the inconveniences of surgery. In the only series published in this age-group, OSASD required closure with hybrid procedures in 21% of children4; although our group is small, we have not needed to employ this technique.
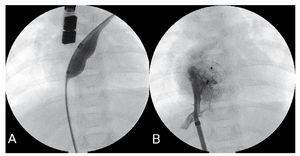
Figure 1. Closure of ostium secundum atrial septal defects (OS-ASD) (patient 3). A: OS-ASD closure with cutting-balloon. B: angiography via previously-deployed 7 Fr sheath, 13 mm device.
During follow-up (mean, 18.7 months), we found no late atrial wall perforation. A review11 of this rare complication found it occurs ≤3 years post-procedure. Patient age or weight were not risk factors. Both mismatching defect and device diameters and anterosuperior defect location have been suggested as risk factors for late of perforation.12
Persistent Ductus Arteriosus
PDA closure was indicated in all four patients because of excessive pulmonary blood flow. In patients 5 and 8, Qp/Qs may have been overestimated due to incorrect blood sampling in the pulmonary artery; however, PDA diameters were 2.5 and 3.2 mm respectively. Patient 4 (Figure 2) was a 40 days old infant girl, with a non-obstructive total abnormal pulmonary veins drainage into coronary sinus and superior vena cava. During cardiac catheterization we found suprasystemic pulmonary hypertension (AP/AO systolic pressure ratio =1.15) and 1.6 mm patent ductus arteriosus; she presented clear clinical improvement following percutaneous duct closure and was operated days later; she evolved well and subsequent examinations showed normalization of pulmonary pressure. In this patient, we decided to use the ductal occluder although the muscular VSD device is considered preferable due to the risk of embolization caused by severe pulmonary hypertension. Despite this, we opted for the ductal occluder because its retention disc is smaller (5 vs 9 mm).
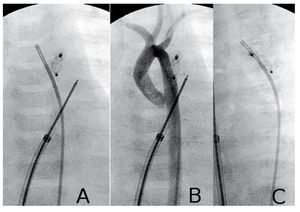
Figure 2.Closure of patent ductus arteriosus (PDA) (patient 4). A and B: device deployed; aortic angiography in posteroanterior projection with and without contrast. C: left oblique projection; note the parallel paths of the occluded ductus and the aorta.
Our results in 15 children aged <1 year are similar to those published by Butera et al13 who reported successful closure in 18 children aged <3 years with PDA treated with the same device.
We have found no aortic obstruction similar to that described in a girl of 2 years who required surgery to remove the device.14 We avoided this complication by using the smallest possible device and also by measuring gradients in the aorta before Amplatzer deployment. During follow-up we found no obstruction in the aortic arch or left pulmonary branch, potential complications given these children were so small. We had no using local vascular complications, despite using sheaths as large as 7 Fr Mullins sheaths in the femoral vein (patients 9 and 14).
Muscular Ventricular Septal Defects
In a series of 20 children aged <1 year, Diab et al5 reported 30% needed a hybrid procedure. In our 4-patient series, none required this. Patient 22, a girl with type I truncus arteriosus, double VSD (one of them muscular apical), had neonatal pulmonary banding. We decided to close the apical m-VSD first because surgical m-VSD closure entails high morbidity and mortality, especially when the location is apical,15 and the surgical approach may need to be from the left ventricle, with the consequent damage to ventricular function.16
Risk of Vascular Lesion
Amplatzer deployment sheaths require venous access and are commercialized by internal diameter size. The largest diameter we used was 7 Fr (patients 2, 3, 9, and 14). In patients with m-VSD (Figure 3), we used 6 Fr sheaths via the internal jugular vein. We believe possible vascular damage caused by such thick sheaths can be minimized by careful handling.
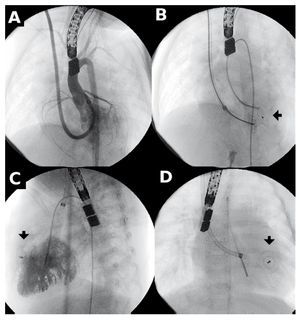
Figure 3. Closure of muscular ventricular septal defect (m-VSD). A: angiography with 6 Fr Mullins sheath, jugular access. A, B, and C: the arrow points to the device before and after deployment.
Correspondence: Dr. F. Prada.
Servicio de Cardiología. Hospital Sant Joan de Déu.
Pg. Sant Joan de Déu, 2. 08950 Esplugues de Llobregat. Barcelona. España.
E-mail: fprada@hsjdbcn.org
Received July 26, 2008.
Accepted for publication December 12, 2008.