Massive pulmonary thromboembolism (PTE) is characterized by sustained hypotension or cardiogenic shock, or both, and has high in-hospital mortality. In addition to hemodynamic and respiratory support, treatment includes anticoagulation and systemic fibrinolysis. Thrombolysis is contraindicated in between one third and one half of patients, mainly due to recent major surgery or trauma, etc., and is unsuccessful in approximately 8% of cases.1 In these situations, the treatment options are surgical embolectomy, in select centers, or alternatively, percutaneous treatment.
In 2013, a protocol was implemented in our hospital for percutaneous intervention in patients with massive PTE and contraindication for thrombolysis. Since then, 24 such patients have been admitted and 5 of them (20%) received percutaneous intervention, performed by the interventional cardiologist (on-call available 24hours). Prior to 2013, intervention had been performed, sporadically, in 3 patients. Thus a total of 8 patients have received attempted percutaneous treatment. Six patients (75%) had cardiorespiratory arrest with pulseless electrical activity. In 3 patients, the initial suspected diagnosis was cardiogenic shock secondary to acute coronary syndrome, with definitive diagnosis of PTE in the catheterization laboratory; in 2 patients, diagnosis was established by transesophageal echocardiography in the operating room; and in the remaining patients, diagnosis was confirmed on CT angiography. The angiographic and catheterization findings are described in the Table. Six patients had thrombotic occlusion of at least 1 pulmonary branch, and the mean pulmonary systolic pressure was 56mmHg (standard deviation, 16mmHg); in 2 patients, the pressures were not recorded due to hemodynamic instability. Five patients received variable doses of thrombolytic, administered as in situ intra-arterial boluses, divided between both pulmonary arteries according to the thrombus size. Given that most patients had a contraindication for systemic thrombolysis, the average dose was approximately one quarter of the systemic dose. Seven patients underwent mechanical treatment with thrombus fragmentation or aspiration (or both), either after intra-arterial thrombolysis if there was no hemodynamic improvement, or simultaneously if the patient's condition was very serious. One patient, with thrombosis in the segmental arteries and normal pulmonary pressure, did not receive percutaneous treatment. Five patients underwent aspiration with an 8 F guidewire-catheter using a Judkins right coronary catheter or multipurpose catheter introduced through an 8.5 F deflectable catheter (Agilis, St Jude Medical), allowing the catheter to be directed to the main affected branches or lobes. In all patients, thrombus was extracted, in variable amounts. Upon thrombus extraction, the lumen of the 8 F guidewire-catheter became occluded and had to be completely withdrawn. Use of the Agilis catheter allowed withdrawal of the 8 F catheters as many times as necessary without losing the position in the pulmonary tree. The Figure shows the deflectable catheter in use. Following mechanical/thrombolytic treatment, pulmonary systolic pressure decreased significantly (final pressure, 37mmHg; standard deviation, 6mmHg; P=.007). The aim of the procedure was to reduce pulmonary pressure and increase systemic pressure and pulmonary oxygenation. One patient died in the catheterization laboratory, although the entire procedure was performed in cardiac arrest (patient 6), and there were 2 in-hospital deaths: 1 patient died of intracranial hemorrhage despite the use of just one quarter of the systemic dose of thrombolytic, and 1 patient had a cardiac arrest in the vascular operating room. This patient, following successful percutaneous treatment of the PTE, required vascular repair to control an arteriovenous fistula hemorrhage caused by femoral vein puncture during catheterization.
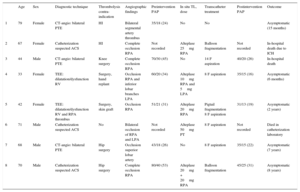
Clinical Data, Angiographic and Catheterization Details, Type of Percutaneous Intervention, In Situ Thrombolysis Dose, and Clinical Outcome
| Age | Sex | Diagnostic technique | Thrombolysis contra-indication | Angiographic findings | Preintervention PAP | In situ TL, dose | Transcatheter treatment | Postintervention PAP | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | Female | CT-angio: bilateral PTE | HI | Bilateral segmental artery thrombus | 35/18 (24) | No | No | Asymptomatic (15 months) | |
| 2 | 67 | Female | Catheterization suspected ACS | HI | Complete occlusion RPA | Not recorded | Alteplase 25mg RPA | Balloon fragmentation | Not recorded | In-hospital death due to ICH |
| 3 | 44 | Male | CT-angio: bilateral PTE | Knee surgery | Complete occlusion RPA | 70/30 (45) | No | 14 F aspiration | 40/20 (26) | In-hospital death |
| 4 | 33 | Female | TEE: dilatation/dysfunction RV | Surgery, hand replant | Occlusion RPA and inferior lobar branches LPA | 60/20 (34) | Alteplase 10mg RPA and 5mg LPA | 8 F aspiration | 35/15 (16) | Asymptomatic (6 months) |
| 5 | 42 | Female | TEE: dilatation/dysfunction RV and RPA thrombus | Surgery, skin graft | Occlusion RPA | 51/21 (31) | Alteplase 20mg RPA | Pigtail fragmentation 8 F aspiration | 31/13 (19) | Asymptomatic (2 years) |
| 6 | 71 | Male | Catheterization suspected ACS | No | Bilateral occlusion of RPA and LPA | Not recorded | Alteplase 50mg PT | 8 F aspiration | Not recorded | Died in catheterization laboratory |
| 7 | 68 | Male | CT-angio: bilateral PTE | Hip surgery | Occlusion superior lobar artery | 43/18 (26) | No | 8 F aspiration | 35/15 (22) | Asymptomatic (7 years) |
| 8 | 70 | Male | Catheterization suspected ACS | Hip surgery | Complete occlusion RPA | 80/40 (53) | Alteplase 20mg + 20mg RPA | Balloon fragmentation | 45/25 (31) | Asymptomatic (8 years) |
ACS, acute coronary syndrome; CT-angio, computed tomography angiography; HI, head injury; ICH, intracranial hemorrhage; LPA, left pulmonary artery; postinterv. PAP, pulmonary artery pressure; PT, pulmonary trunk, PTE, pulmonary thromboembolism; RPA, right pulmonary artery; RV, right ventricle; TEE, transesophageal echocardiography; TL, thrombolytic.
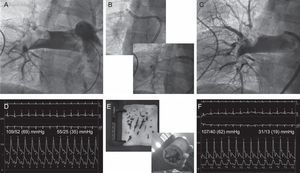
Patient 5. A: Angiography showing a large thrombus lodged in the right pulmonary branch. B: Thrombus aspiration with a deflectable catheter that allowed the passage of an 8 F guidewire-catheter through the thrombus. C: Final angiography, after mechanical/fibrinolytic treatment, showing residual thrombus but good distal perfusion. D: Baseline aortic and pulmonary pressures. E: Gross samples of aspirated thrombus. F: Final aortic and pulmonary pressures.
Mortality from massive PTE is very high (approximately 30%) and is 3 to 7 times higher in patients who have undergone cardiopulmonary resuscitation. In our study, mortality was 33%, taking into account that 1 patient did not undergo intervention and that another patient underwent the entire procedure in prolonged cardiac arrest. Currently, there are no randomized studies comparing endovascular treatment with thrombolysis or with surgical embolectomy, and therefore the existing evidence comes from observational studies. A systematic analysis that included 594 patients with massive PTE (from 35 studies) found a success rate, defined as resolution of hypoxia, hemodynamic stabilization, and discharge from hospital, of 85.5% (between 40% and 100% depending on the series), and a major complication rate of just 2.4%.1 This difference in mortality compared with our study is probably due to the profile of the patients included, considering that most patients in our series had had a cardiac arrest. In addition to reducing the thrombotic load by thrombus fragmentation and aspiration, endovascular treatment has the advantage of being able to administer the thrombolytic in situ, increasing its effectiveness and reducing the risk of hemorrhage because low doses can be administered. Fragmentation and aspiration were performed only in the main arteries and lobar arteries, not in the segmental arteries, and the procedure was ended as soon as hemodynamic and respiratory improvement were obtained, independently of the angiographic result. There was 1 serious procedural complication, which was a femoral arteriovenous fistula requiring vascular repair, and the patient died of a probable rethrombosis when anticoagulation was withheld.
This single-center study shows that percutaneous treatment of massive PTE is effective and offers an alternative to surgical embolectomy when fibrinolysis is contraindicated or has failed. In Spain, urgent percutaneous treatment of PTE could be easily implemented in a large number of hospitals with on-call teams of interventional cardiologists or radiologists.