Keywords
INTRODUCTION
The Blalock-Taussig systemic-pulmonary shunt (SPS) is a palliative surgical procedure that continues to be useful in the initial management of certain congenital heart diseases with reduced pulmonary blood flow. On occasion, it is not possible to close these shunts in a subsequent palliative or corrective surgical procedure because of technical limitations. In these cases, percutaneous closure is an attractive alternative. Likewise, the SPS created in patients with pulmonary atresia and intact ventricular septum who have undergone pulmonary valvotomy during the neonatal period can be closed by means of a catheter intervention as it improves right ventricular distensibility.1 However, as the literature has demonstrated highly variable results with the percutaneous closure of these shunts,2-7 we were encouraged to report our experience with this mode of treatment involving arterial embolization using Gianturco coils.
METHODS
We reviewed the medical records of the patients who underwent attempted percutaneous closure of SPS in our departments between January 1996 and August 2006. The indication for closure was the need to reduce the risk of endocarditis and prevent systemic ventricular volume overload.
The procedures were carried out, following informed consent, with general anesthesia. Femoral artery and venous access were obtained using a 5-French and 6-French introducer, respectively. Intravenous heparin (100-150 IU/kg body weight) and antibiotic prophylaxis with cephalothin were administered. Selective angiography of the SPS was performed in standard projections by means of the injection of the contrast medium into the aortic origin of the anastomosis in order to determine the diameter and length and to detect the presence of stenosis in its interior. In the patients with pulmonary atresia and intact ventricular septum, the tolerance to the occlusion of the Blalock-Taussig shunt was evaluated approximately one year after the pulmonary valvotomy performed during the neonatal period. A balloon catheter was inflated within the SPS via the artery, and the foramen ovale was closed via the vein using a Berman catheter.1After 15 to 20 minutes, those patients exhibiting normalization of the arterial oxygen saturation and with no decrease (>20%) in cardiac output and arterial blood pressure were considered to be tolerant. In cases of intolerance, the SPS was not closed.
The coil was selected considering a loop diameter 1 to 2 mm greater than the maximum diameter of the Blalock-Taussig shunt, with a length determined by the formation of 2 to 3 loops. AJudkins right coronary catheter was placed in the distal third of the shunt and the coil was advanced using a 0.035-inch guide wire, while the position of the catheter was sustained. In some patients, a balloon catheter with 1:1 ratio with respect to the diameter of the isolateral pulmonary artery was placed transvenously in order to reduce the blood flow through the SPS and prevent embolization of the coil. The presence of a significant shunt after the first coil was implanted determined the need for additional coils, with a loop having a diameter 1 mm smaller than that of the first coil.
The procedure was considered to have been successful when proper positioning was achieved, in the absence of embolizations and of shunt, verified by means of angiography performed 10 minutes after release of the coil. Follow-up of the patients was carried out to rule out shunt recurrence and/or embolization.
RESULTS
Percutaneous closure was attempted in 10 patients (7 boys) with a modified Blalock-Taussig shunt and the same number of fistulas (Table 1). The median age and weight were 2.8 years (range, 5 months to 14 years) and 12 kg (range, 4 to 50 kg), respectively. Five patients had pulmonary atresia with intact ventricular septum, and underwent radiofrequency pulmonary valvotomy. Three had tetralogy of Fallot and 2, a univentricular heart; in these patients, it was not possible to close the Blalock-Taussig shunt during subsequent definitive complete repair or palliation.
The maximum diameter of the shunt was 3.35 (0.47) mm and the minimum diameter, 2.55 (0.89) mm. Amedian of one coil was implanted per patient, successfully in every case, and with a rate of immediate occlusion of 100% (Figures 1 and 2). There were no complications. Two patients underwent pulmonary stent placement during the same procedure (cases 6 and 10), one in left branch and one in right branch. Distal flow was occluded using an angioplasty balloon in another 2 patients (cases 4 and 8) (Figure 1).
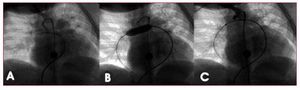
Figure 1. Coil implantation using a technique with balloon support. A: angiography in right anterior oblique projection showing the patency of the Blalock-Taussig shunt on the right, with no stenosis in its interior. B: inflated balloon in right branch, simultaneous to coil implantation. C: angiography following implantation showing complete shunt occlusion.
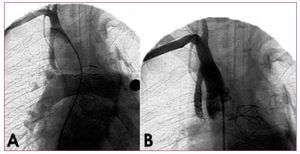
Figure 2. Angiography of the Blalock-Taussig shunt prior to and after implantation. A: a 5-mm systemic-to-pulmonary artery shunt between the innominate and right pulmonary arteries. B: complete closure following embolization with several coils.
During follow-up (median, 3 years), chest radiography and echocardiography were performed to ensure that the devices remained well positioned and that the shunt did not recur.
DISCUSSION
The first case in which percutaneous embolization was performed was reported by Culham et al in 1981. They employed 2 Gianturco coils cut to size, one of which embolized toward the pulmonary circulation.2 Since then, a variety of techniques and devices have been utilized, including detachable balloons, Gianturco coils, controlled-release coils, Rashkind umbrellas, the Grifka vascular occlusion device and Amplatzer occluders.3-8 The limitations of most of them are the cost, the use of demanding techniques, large introducer sheaths and limited flexibility in the release system.8
Few studies involving coherent series of percutaneous closure of Blalock-Taussig shunts have been published.7,9,10 Burrows et al7 obtained a low success rate in their group of 17 patients due to incomplete closure (58%) and a high rate of migration of the Gianturco coils toward the pulmonary arteries. These authors attribute their results to the absence of stenosis and the high flow rate through the shunts. Perry et al9 reported similar outcomes in 14 patients, with the additional description of hemolysis in a case in which there was significant residual shunt, resulting in low degrees of efficacy and safety. In contrast, Moore et al11 reported successful results, with no complications, in a series of 18 patients in whom they used controlled-release coils for the shunts in which there was no stenosis in their interior and Gianturco coils in those cases in which there was stenosis or there had been previous implantation of a stent in the pulmonary arteries. In 2 of our cases, we also took advantage of the presence of the stents in the pulmonary arteries in order to reduce the risk of embolization.
In the present series, the retrograde approach was used to implant the Gianturco coils in every case, as it is a simple and inexpensive option that is available in our institution. Although this modality does not have a controlled-release system, the technique can be optimized by the proper selection of the coil. A diameter 1 to 2 mm greater than the diameter of the Blalock-Taussig shunt is recommended in order to take advantage of the radial force of the devices to secure them in the shunt, where they form a pack. Greater diameters would result in a straight configuration of the device, which would increase the risk of protrusion and/or migration, and the coil would not perform its function of generating thrombosis. In addition, the protrusion of the coil loops toward the subclavian artery is avoided with a good catheter stabilization technique and with the proper choice of the length of the coil, ensuring the complete formation of only two to three loops within the shunt. In cases in which there is no stenosis, distal flow should be controlled by means of an angioplasty balloon, as we did in two of our patients.
In conclusion, percutaneous closure of a Blalock-Taussig SPS by means of retrograde arterial embolization with Gianturco coils is a feasible, safe, effective, and inexpensive treatment modality. It can be performed in cases in which surgical closure is technically impossible or in patients who do not require surgery of some other type. Moreover, in cases in which there is no stenosis within the SPS, an angioplasty balloon can be inserted into ipsilateral pulmonary artery to reduce the risk of embolization.
Correspondence: Dr. C.A.C. Pedra.
Sección Médica de Intervenciones en Cardiopatías Congénitas. Instituto Dante Pazzanese de Cardiologia.
Avda. Dr. Dante Pazzanese, 500. CEP 04012-180. São Paulo, SP, Brasil.
E-mail: cacpedra@uol.com.br
Received December 13, 2006.
Accepted for publication February 12, 2008.