Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is the main circulatory assistance technique for cardiogenic shock in the pediatric population.1 To avoid congestion and distension of the left heart and consequent pulmonary edema, a drainage system is required to facilitate ventricular rest and recovery. One such drainage technique is percutaneous atrial septostomy (PAS).2,3
In our hospital, urgent PAS is performed as standard for all patients with severe ventricular dysfunction who require VA-ECMO support. We report a series of 16 patients who were admitted to our hospital between 2015 and 2020 in cardiogenic shock and requiring VA-ECMO support. Peripheral cannulation of the neck (right jugular vein and carotid artery) was used for ECMO in all patients, with subsequent surgical repair in most. Once stabilized on ECMO, the patients were transferred to the cardiac catheterization laboratory for PAS. During the procedure, we took the opportunity to take an endomyocardial biopsy in selected patients.
The PAS technique is carried out, if there is vascular availability, with double venous access. Via one access line, a snare is placed in the inferior vena cava; via the other access line, the PAS material is placed, which is passed through the snare.4 If the foramen ovale is not patent, PAS is performed with a Brockenbrough needle under transesophageal echocardiographic guidance (video 1 of supplementary data). The selection of the type and size of stent is influenced by several factors: left atrial pressure (the main factor), inotropic support, and aortic valve opening. With higher left atrial pressure, more inotropic support, and a closed valve, the diameter of the stent should be closer to half the length of the septum; in the opposite situation, closer to a third. In target diameters of up to 10mm, a 10 × 19 Palmaz Genesis stent (Cordis Corp, USA) is used; in larger diameters, a Cheatham-Platinum stent (NuMED Inc, USA) is used. The snare is moved until it touches the interatrial septum, marking where the center of the final position of the stent (video 2 of the supplementary data); when it is tensed during inflation, this gives the stent a slight hourglass shape and better stability (video 3 of the supplementary data). At the end of the procedure, the absence of a hemodynamic gradient between the atria is checked to confirm that the correct stent diameter has been chosen.
Statistical analysis was performed using R Core Team version 2016 (R: A language and environment for statistical computing, R Foundation for Statistical Computing, Austria). Continuous variables are presented as median (range), and categorical variables, as frequency. The Wilcoxon test was used for the difference in left atrial pressures after the procedure. P values < .05 were considered statistically significant.
The characteristics of the patients and the procedure are described in table 1 and table 2. An interatrial stent was inserted in all patients except 1 neonate, who underwent PAS with balloon only, as the left atrial pressures were not significantly high and there was a ventricular septal defect. In the same procedure, we took an endomyocardial biopsy for diagnostic purposes from 11 patients; this was not done in 5 due to a low probability of myocarditis or high risk due to very low weight. One limitation is that we did not keep a record of all the pressures measured, so information on initial final values of left atrial pressure was available for review in only 8 patients. In these patients, a significant decrease was confirmed, with a median −16.5mmHg (95% confidence interval, 23-5.5mmHg; P=.02). Based on experience from earlier series, showing a reduction in ECMO time associated with early PAS,5 our protocol recommends urgent PAS after starting ECMO to favor early resolution of pulmonary edema, faster ventricular recovery, and shorter duration of ECMO, with a median 5.8 (3-12.9) hours between starting ECMO and PAS completion. All the patients had pulmonary edema, which resolved after PAS. At 14 days and 62 days, 2 patients returned to the catheterization laboratory due to recurrent pulmonary edema to rule out stent stenosis, and low hemodynamic gradients were observed (2-3 mmHg), with high biventricular filling pressures that explained the recurrence of edema. The stent was dilated to eliminate the gradient, then diastolic failure and venous congestion were treated, with clinical improvement. No complications associated with the procedure were observed in any of the patients.
Overall characteristics of the patients and procedure
| Patient characteristics | Procedure details | ||||
|---|---|---|---|---|---|
| Sex | Men | 6 | Access | Double access | 9 |
| Women | 10 | Single access | 7 | ||
| Age | 6.8 years [10 d-15 y] | Septum length, mm | 28 [11-49] | ||
| Weight, kg | 17.5 [2.5-77] | Stent implanted | 15 | ||
| Diagnosis | Myocarditis | 8 | Type of stent | PG 19×10 | 7 |
| Inherited DCM | 4 | PG 25×10 | 1 | ||
| Idiopathic DCM | 4 | CP 34 mm | 5 | ||
| CP 28 mm | 2 | ||||
| Outcome | Recovery | 8 | Stent diameter, mm | 11 [10-20] | |
| Transplant | 6 | ||||
| Death | 2 | ||||
| ECMO, days | 18 [7-78] | Endomyocardial biopsy | 11 | ||
| Time admission-ECMO, h | 11.5 [3-288] | Dilatation of stent | 2 | ||
| Time ECMO-stent, h | 5.8 [3-12.9] | Procedural time, min | Stent and biopsy | 101 [68-147] | |
| Stent | 67 [29-137] | ||||
| Fluoroscopy time, min | Stent and biopsy | 34 [17-63] | |||
| Stent | 19 [10-43] | ||||
CP, Cheatham-Platinum stent (NuMED Inc, USA); DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenator; LAP, left atrial pressure; PG, Palmaz Genesis stent (Cordis Corp, USA).
Values are expressed as No. (%), mean±standard deviation or median [range].
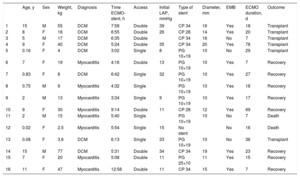
Individual characteristics of the patients and procedure
| Age, y | Sex | Weight, kg | Diagnosis | Time ECMO-stent, h | Access | Initial LAP, mmHg | Type of stent | Diameter, mm | EMB | ECMO duration, d | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | M | 55 | DCM | 7:58 | Double | 39 | CP 34 | 16 | Yes | 18 | Transplant |
| 2 | 8 | F | 18 | DCM | 6:55 | Double | 26 | CP 28 | 14 | Yes | 20 | Transplant |
| 3 | 5 | M | 17 | DCM | 6:35 | Double | CP 34 | 16 | No | 7 | Transplant | |
| 4 | 9 | F | 40 | DCM | 5:24 | Double | 35 | CP 34 | 20 | Yes | 78 | Transplant |
| 5 | 0.16 | F | 4 | DCM | 3:02 | Single | 8 | PG 10×19 | 10 | No | 29 | Transplant |
| 6 | 7 | F | 19 | Myocarditis | 4:16 | Double | 13 | PG 10×19 | 10 | Yes | 7 | Recovery |
| 7 | 0.83 | F | 8 | DCM | 6:42 | Single | 32 | PG 10×19 | 10 | Yes | 27 | Recovery |
| 8 | 0.75 | M | 9 | Myocarditis | 4:32 | Single | PG 10×19 | 10 | Yes | 18 | Recovery | |
| 9 | 2 | M | 13 | Myocarditis | 3:34 | Single | 9 | PG 10×19 | 10 | Yes | 17 | Recovery |
| 10 | 9 | F | 30 | Myocarditis | 9:14 | Double | 11 | CP 28 | 12 | Yes | 69 | Recovery |
| 11 | 2 | M | 15 | Myocarditis | 5:40 | Single | PG 10×19 | 10 | No | 7 | Death | |
| 12 | 0.02 | F | 2.5 | Myocarditis | 5:54 | Single | 15 | No stent | No | 16 | Death | |
| 13 | 0.08 | F | 3.6 | DCM | 6:13 | Single | 33 | PG 10×19 | 10 | No | 36 | Transplant |
| 14 | 15 | M | 77 | DCM | 5:31 | Double | 34 | CP 34 | 19 | Yes | 23 | Recovery |
| 15 | 7 | F | 20 | Myocarditis | 5:08 | Double | 11 | PG 25×10 | 11 | Yes | 15 | Recovery |
| 16 | 11 | F | 47 | Myocarditis | 12:58 | Double | 11 | CP 34 | 15 | Yes | 7 | Recovery |
CP, Cheatham-Platinum stent (NuMED Inc., USA); DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenator; EMB, endomyocardial biopsy; F, female; LAP, left atrial pressure; PG, Palmaz Genesis stent (Cordis Corp.,USA); M, male.
At follow-up (median 16.8 [0.3-54.2] months), of the 8 patients who recovered ventricular function, we observed mild right heart dilatation in 5 patients—median Z-score +2.3 (0.4-4.3)—secondary to a left-to-right shunt produced by the interatrial stent; so far this has not required closure. As this group comprised essentially patients with a defect diameter of 10 mm, which over time will tend to get smaller due to neointimal growth in the stent, it is reasonable to assume that at least some of the defects may become insignificant over time and will not require percutaneous closure, although, given the support guaranteed by the stent, it seems likely such a procedure would be technically very simple.
The percutaneous creation of a nonrestrictive atrial septal defect is a safe and effective solution to the problem of left heart drainage in patients with cardiogenic shock requiring VA-ECMO, and also allows endomyocardial biopsy during the same procedure when indicated.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.01.010