Keywords
INTRODUCTION
Porstmann et al were the first to report the percutaneous closure of a patent ductus arteriosus (PDA).1 During the 1960s, Rashkind designed the "double umbrella" device, which was later used extensively.2-5 In the 1990s Gianturco coils were used6 and then Jackson coils with a controlled release system.7
Later on, devices appeared with different anchoring and release systems, such as the Sideris button,8 the Grifka bag9 and the Amplatzer device.10 Another recent device is the Nit-Occlud (NOc), which has undergone several modifications in material, release system and configuration since it was first designed,11,12 with the latest version being in use since 2001. We report our experience with this device.
METHODS
From May 2003 to July 2006 we undertook percutaneous closure with the NOc in 28 patients (13 male) who had a clinical and echocardiographic diagnosis of isolated PDA (one involving post-surgical recanalization).
The ages of the patients ranged from 0.5 to 21 years (median, 1.8 years), and their weight ranged from 5.9 to 64 kg (median, 10.9 kg). The patients were managed clinically, radiologically and by echocardiography.
The device is composed of a coil of nitinol with no thrombogenic material which, on implantation, acquires a double cone structure like an hourglass. The pulmonary end adopts a reverse form, endowing it with a safer anchoring. The device is premounted on a controlled release system, which can be used with 4 to 6 Fr sheaths. The device is compatible with magnetic resonance imaging studies.13
The statistical analysis was done with the program SPSS, version 12.0.
Procedure
Under general anesthesia, the right femoral vessels were approached by the Desilets-Hoffman method, using 3 or 4 Fr in the artery and 4 to 6 Fr sheaths in the vein. Heparin was administered at a dose of 100 U/kg. After the hemodynamic study, aortography was performed to define the ductal morphology and measure its ends.
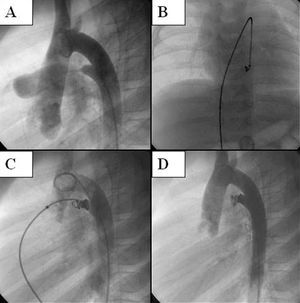
Due to the difficulty to catheterize the PDA from the pulmonary end in four patients (14.2%), a guide wire was advanced via the arterial end, creating an arteriovenous loop. The device was advanced from the femoral vein in all cases. The correct position of the occluder was verified by aortography and, if necessary, it was repositioned, as the device allows this to be done. A post-implant control aortography was done 10 min after its release (Figure). Prophylactic cefazoline was given.
Figure. Angiographic sequence of the implantation of the Nit-Occlud device in patent ductus arteriosus. A. Lateral arteriography showing the ductal morphology. B and C. Presenting and positioning of the device. D. Implanted device, with no immediate residual shunt.
RESULTS
Implant of the NOc device was possible in all cases; its size was chosen in accordance with the nomogram indicated by the manufacturer.
The mean pulmonary artery pressure ranged from 13 to 28 mm Hg (mean, 17.6 mm Hg). The pulmonary and the aortic ampulla diameters were between 0.7 and 4 mm (mean, 1.8 mm) and between 2.8 and 12.1 mm (mean, 6.5 mm), respectively (Table 1).
All types of ductal forms according to the classification of Krichenko et al14 were found. The immediate residual shunt was classified as follows: trace, if the contrast was slightly opaque at the ductal pulmonary end; mild, if the pulmonary artery was stained without outlining its valve; moderate, if the whole valvular plane was outlined.13 The rates of immediate residual shunt were: absent in 53.5%, trace in 10.7%, mild in 17.8%, and moderate in 17.8%. The patient admission time was 24 h in all cases.
No association was found between the magnitude of the initial residual shunt and the occlusion of the PDA at 24 h, as in some patients with a moderate immediate residual shunt, it had closed by the following day and in other patients with a mild residual shunt it remained patent.
The rate of post-implant occlusion of the NOc is shown in Table 2. Five patients underwent the procedure less than six months before the time of writing and three of them have no residual shunt. The follow-up of the patients ranged from 0.5 to 38 months (median, 20.5 months). No complications were recorded during the procedure or the follow-up.
DISCUSSION
The reduction in the profile of the implanting system has enabled treatment of smaller patients with a lower rate of complications during the vascular access or positioning maneuvers. Accordingly, as the NOc permits sheaths of 4-6 Fr, it is especially useful in children younger than one year of age, as well as proving versatile with different ductal morphologies, as it can be withdrawn and repositioned.
The manufacturer does not recommend its use in PDA with a minimum diameter in excess of 6 mm. This disadvantage does not apply to the Amplatzer device.15 The largest pulmonary diameter in our series of patients was 4 mm, with a moderate immediate residual shunt that was mild at the six-monthly control.
As with other groups,13,16 we had a considerable rate of immediate residual shunt, which was markedly reduced at 24 h. Its persistence had no hemodynamic repercussion in any patient.
In conclusion, in our experience the NOc proved useful for the percutaneous closure of PDA, independently of its morphology. The rate of effectiveness was comparable to that reported with other devices used with the same aim.17-20 Our results show that, using the adequate device for the size of the PDA, the magnitude of the immediate residual shunt was not an adverse indicator of later occlusion.
Correspondence: Dr. R. Gamboa.
Av. Belgrano, 1746 Piso 5.°.
Buenos Aires C1093AAS. Argentina.
E-mail: rgamboa@ffavaloro.org
Received April 27, 2006.
Accepted for publication November 30, 2006.