Major international practice guidelines recommend the use of a combination of 4 medication classes in the treatment of patients with heart failure with reduced ejection fraction (HFrEF) but do not specify how these treatments should be introduced and up-titrated. Consequently, many patients with HFrEF do not receive an optimized treatment regimen. This review proposes a pragmatic algorithm for treatment optimization designed to be easily applied in routine practice. The first goal is to ensure that all 4 recommended medication classes are initiated as early as possible to establish effective therapy, even at a low dose. This is considered preferable to starting fewer medications at a maximum dose. The second goal is to ensure that the intervals between the introduction of different medications and between different titration steps are as short as possible to ensure patient safety. Specific proposals are made for older patients (> 75 years) who are frail, and for those with cardiac rhythm disorders. Application of this algorithm should allow an optimal treatment protocol to be achieved within 2-months in most patients, which should the treatment goal in HFrEF.
Keywords
Heart failure (HF) is a major cause of hospitalization, morbidity and mortality, notably in older patients, with an estimated worldwide prevalence of around 2%.1 Nonetheless, appropriate management effectively prevents disease aggravation, acute decompensations and saves lives,2 although optimization is rarely obtained in real life. New international practice guidelines have recently been published,3,4 which recommend the use of a combination of 4 medication classes as the platform therapy for HF with reduced ejection fraction (Class I recommendation). These classes are certain beta-blockers (BB), angiotensin receptor-neprilysin inhibitors (ARNIs) or angiotensin-converting enzyme inhibitors (ACEIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter 2 inhibitors (SGLT2Is). However, the optimal approach to introducing this guideline-directed medical therapy (GDMT) in a given patient remains a matter of debate.5–12
Major factors limiting the introduction and titration of HF medications include worsening renal function,13 low blood pressure,14 and low heart rate.15 Concomitant initiation of multiple drugs which carry a risk of these adverse events can be a hurdle, which needs to be considered when choosing a therapeutic strategy. Likewise, rapid treatment optimization, ideally within a 2-month timeframe, is also a challenge.
In the present article, we propose a pragmatic approach designed to be easily applied by practicing physicians in routine clinical practice. We have attempted to take into account recent guidelines, the notion of early implementation and its difficulties, and the need to tailor management to individual patient requirements. This position statement was prepared through a collaboration among experts of the Heart Failure Working Group of the French Society of Cardiology, with the aims of promoting treatment optimization and facilitating the practical implementation of the ESC HF guidelines.4
FACTORS TO CONSIDER WHEN DESIGNING AN ALGORITHM FOR MEDICATION OPTIMIZATION IN CHRONIC HEART FAILURE WITH REDUCED EJECTION FRACTIONPharmacological considerationsThe various classes of GDMT target different pathophysiological mechanisms: ARNIs/ACEI and MRAs inhibit the renin-angiotensin-aldosterone system,16 with ARNIs also specifically blocking the degradation of natriuretic peptides and other vasoactive hormones.17 BBs principally target the autonomic nervous system, but also inhibit renin synthesis.18 Finally, SGLT2Is were originally developed to prevent glucose and sodium reabsorption in the kidney, but their beneficial effects in HF probably involve extrarenal mechanisms that require further elucidation.19 From a pharmacological perspective, it appears reasonable to target the maximum number of physiopathological mechanisms in parallel, rather than attempting to achieve maximal inhibition of a single pathway.
Efficacy considerationsHistorically, GDMT classes were introduced sequentially, following demonstration of their efficacy from well-designed randomized clinical trials spanning a period of more than 30 years. For this reason, each novel class has usually been evaluated against a placebo while being used in conjunction with previously available medications. Direct head-to-head comparative studies have not generally been performed, except in the case of ARNIs vs ACEIs.20
To address the limited data from direct comparisons, the relative efficacy of different HF treatments has been addressed in a recent meta-analysis of 75 randomized clinical trials.21 Unsurprisingly, the most effective option in reducing all-cause death vs placebo was the concomitant use of an ARNI, a BB, an MRA and a SGLT2I.21 Importantly, there is no evidence for interactions between classes of HF medication and the available data strongly suggest that each class has an independent impact on clinical outcomes, regardless of the other drugs used to treat the patient.8,22
As well as the absolute treatment effect sizes of the various medication classes, the sequence of their introduction. For example, a recent study using data from 6 pivotal trials to model different treatment sequences10 reported some differences in mortality outcomes between sequences. However, the most important factor in reducing mortality was the rapidity of treatment up-titration.10
Early introduction of therapy following diagnosis or an acute exacerbation is recommended to optimize prognosis. Post hoc findings from recent trials have consistently demonstrated rapid risk reduction following treatment initiation. For example, in a large randomized trial comparing sacubitril/valsartan with enalapril,20 and in recent trials of SGLT2i,23,24 rehospitalizations were significantly reduced within 1 month of treatment initiation. Recently, in the STRONG-HF trial,25 900 hospitalized patients were randomized to management either with usual care or high-intensity care. The latter group received rapidly up-titrated 4-drug therapy to achieve optimal doses within 2 weeks of discharge. This approach was feasible and safe, and the trial demonstrated that rapid titration of GDMT significantly reduced the risk of 180-day all-cause death or HF hospitalization.25 Acute hospitalizations provide a window of opportunity to initiate and optimize treatments for HF, and this window of opportunity will be lost if patients are discharged untreated.
Safety considerationsThe main adverse events limiting GDMT optimization are low blood pressure, low heart rate, impaired renal function and electrolyte disturbances, mainly hyperkalemia with MRAs.26 In this respect, appear to have the best safety profile as their effect on blood pressure is minimal and they do not generally cause orthostatic hypotension in patients without hyperglycemia (which can easily be rectified with intensification of other treatments for diabetes).27 However, SGLT2Is may increase the risk of ketoacidosis,28 especially if the patient becomes hemodynamically unstable and goes into shock. In such cases, SGLT2I treatment should be delayed or discontinued.27 These drugs may also increase the risk of urinary infections in patients with a urinary catheter in place.28
Low blood pressure is principally an issue for BBs, ACEIs, and ARNIs. Impaired renal function, due to reversible hemodynamic effects, is observed with MRAs, ACEIs and ARNIs.13,29 While SGLT2I treatment may lead to a small early rise in serum creatinine, these drugs provide significant renal protection in the mid-term.13 Of note, little information is available on medication risk in patients with very severe HF (ejection fraction <25%), who may require more conservative management. Difficulties related to low blood pressure and impairment of renal function can be attenuated with recently proposed management algorithms.14,29
Another factor influencing the speed of GDMT optimization is the persistence of congestion, which is associated with poor prognosis after discharge.30 Apart from BB, which may need to be introduced later or titrated more slowly, persistent congestion should not prevent GMDT optimization prior to discharge. Indeed, ACEIs, ARNIs and SGLT2Is have been shown to improve decongestion.31–33 However, the question of managing congestion is a complex one and deserves a lengthier discussion elsewhere.
ComorbiditiesComorbidities are frequent in HF, especially diabetes, chronic respiratory diseases, and chronic kidney disease.1 Many of the medications used for the treatment of HF are also effective in these other conditions or diseases, such as hypertension or long-standing coronary artery disease. In patients with diabetes, the use of SGLT2Is in HF may improve glycemic control as well as improving cardiac function. This class of drug has also more recently been shown to reduce disease progression and mortality in patients with chronic kidney disease.34 Moreover, ARNIs have also been shown to improve diabetes control35 and renal function in patients with HF.
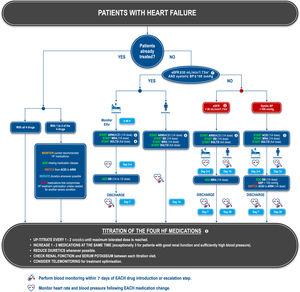
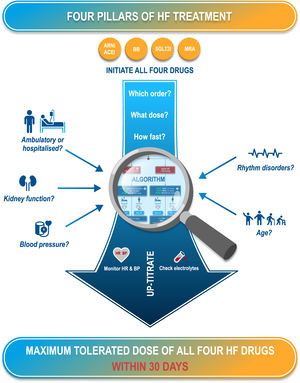
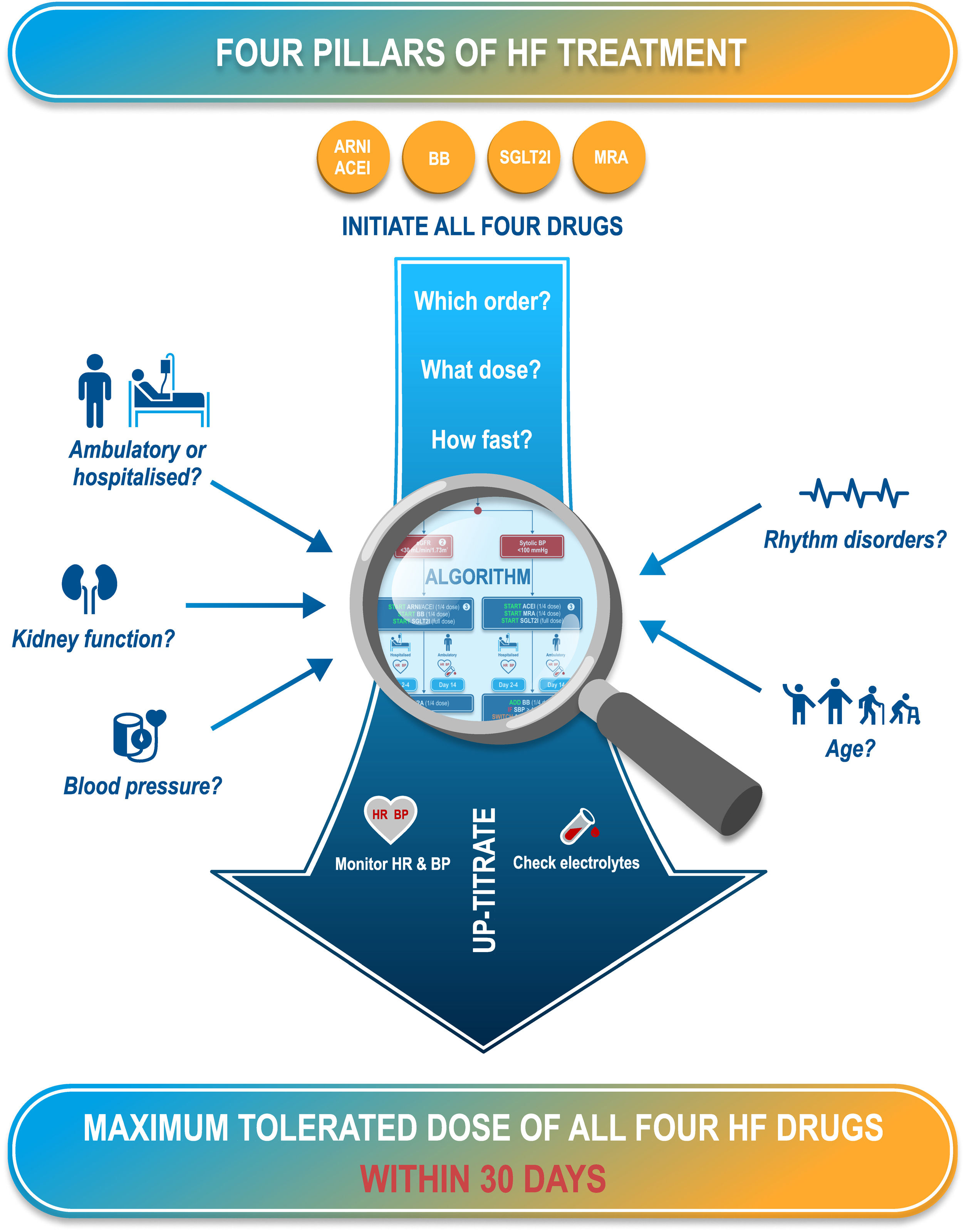
PROPOSED ALGORITHM FOR OPTIMIZATION OF HEART FAILURE MEDICATIONOn the basis of the available evidence, we propose an algorithm for the initiation or optimization of treatments for HF (figure 1). Our goal is to provide a simplified approach to patients’ heterogeneous clinical presentations, focusing on key factors that may influence drug tolerability (figure 2). The proposed algorithm can be applied to most patients. However, we acknowledge that there are other patient groups with more complex clinical presentations. In these cases, our algorithm may be adapted for a more sophisticated comorbidity-based approach.36 It should be noted that we focus here on a practical approach to optimize GDMT, and do not discuss other important aspects of HF management, such as second-line treatments, nonpharmacological management, and the management of comorbidities.
Proposed treatment algorithm for patients with heart failure. 1. In patients with an eGFR between 30 and 40mL/min/1.73m2, kidney function and serum electrolytes should be monitored more closely. 2. Particular attention should be paid to regular serum potassium monitoring in this group. 3. ARNIs are to be preferred to ACEIs, as this will allow more rapid treatment optimization. However, ACEIs can be used as an alternative, notably if ARNIs are contraindicated. A ¼ dose of ARNI sacubitril/valsartan corresponds to 24/26mg bid and a ½ dose to 49/51mg bid. 4. In patients with ventricular tachycardia or premature ventricular contractions, a BB should be preferred to an ACEI as a first step. Caution should be exerted in patients with either very low BP or clinical instability. 5. Titration should not be considered definitive, and medication should be reassessed at each follow-up visit according to the patient's general health status. Even in patients whose ejection fraction improves after treatment, guideline-directed medical therapy should be pursued.
ACEI, angiotensin-converting enzyme inhibitor; AE, adverse events; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blockers; BP, blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; MRA, mineralocorticoid receptor antagonist; SGLT2I, sodium-glucose like transporter type 2 inhibitor; VT, ventricular tachycardia.
Central illustration. A proposed algorithm for the introduction and optimization of the 4 medications in guideline-directed medical therapy based on the factors influencing drug tolerability and providing recommendations for monitoring and titration to achieve optimal dosing within 30 days. ACEI, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blockers; BP, blood pressure; HR, heart rate; MRA, mineralocorticoid receptor antagonist; SGLT2I, sodium-glucose like transporter type 2 inhibitor.
The underlying principles of this algorithm (figure 2) are the following:
- a)
To ensure that all 4 recommended GDMT classes are initiated as early as possible to establish effective therapy, even at a low dose. This is considered preferable to starting fewer medications at a maximal dose, and was identified as the most effective strategy in the network meta-analysis.21
- b)
To ensure that the intervals between the introduction of different medications and between different titration steps are as short as possible, consistent with ensuring patient safety. Again, rapid titration has been shown to provide mortality benefits in the STRONG-HF trial.25
- c)
To ensure that the time from first treatment initiation to reaching the target dose for all 4 GDMT components4 does not exceed 30 days and that the patient is stabilized on optimal medication dosing within 2 months.
The algorithm proposed is a general one, developed for use in all patients with HF, except for 2 specific populations, who may require different treatment strategies. The needs of these patients are addressed separately below. This concerns:
- a)
Patients aged> 75 years who are frail (defined as a Triage Risk Screening Tool [TRST] score ≥ 2)37,38
- b)
Patients with certain cardiac rhythm disorders
The algorithm proposes specific management pathways depending on the number of current HF medication classes used, on whether the patient is hospitalized or not, and on the presence of low blood pressure (systolic blood pressure <100mm Hg) or impaired renal function (estimated glomerular filtration rates [eGFR] <30 mL/min/1.72 m2).
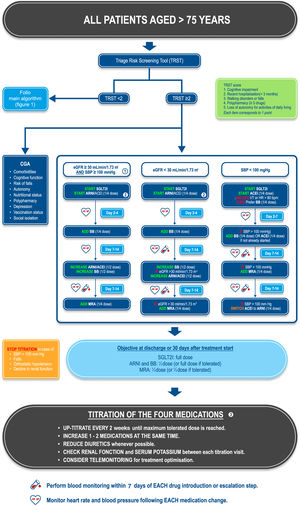
Management of patients aged> 75 yearsThe prevalence of HF rises steeply with age, reaching between 15% and 20% in individuals aged ≥ 80 years.39 These patients are more likely to develop adverse events to medication, are likely to already be polymedicated, and to have unfavorable prognostic factors, such as renal failure, cognitive disorders, a risk of falls, and malnutrition. These factors should be considered when deciding how to optimize treatment in these patients. Moreover, frailty is highly common in older people with HF (45%),40 which increases the risk of mortality and hospitalizations.41
All patients aged> 75 years should undergo frailty assessment using validated frailty scales. We recommend using the TRST,38 which is a very simple and rapid test that includes multiple dimensions of frailty. Recently a cardiology/geriatrics consensus group proposed the use of the TRST as a frailty screening tool in the cardiology setting.37 Patients with a TRST score ≥ 2 should be considered frail and treated more conservatively. However, other frailty scales can also be used, including the FRAIL scale,42 the Clinical Frailty Score,43 or the Fried criteria,44 although these scales cover fewer dimensions of frailty than the TRST.
In frail patients, treatment escalation should be more gradual than that proposed in the general HF treatment algorithm. Starting doses of all drug classes should be ¼ the full dose, with the exception of SLGT2Is, which can be given at the full dose. The dosing interval should be extended to 1 to 2 weeks between each change in medication and the dose increased in incremental steps of a ¼ dose. The final dose of all medications will more often be below the usual targets specified in treatment guidelines for all drugs, apart from that of SGLT2Is. Nonetheless, titration to maximally tolerated doses in the elderly should be attempted whenever possible. An algorithm illustrating the proposed treatment escalation sequences for frail patients> 75 years old according to their baseline blood pressure and kidney function status is provided in figure 3.
Proposed treatment algorithm for patients aged> 75 years with heart failure. 1. In patients with an eGFR between 30 and 40mL/min/1.73m2, kidney function and serum electrolytes should be monitored more closely. 2. ARNIs are to be preferred to ACEIs, as this will allow the treatment to be optimized most rapidly. However, ACEIs can be used as an alternative, notably if ARNIs are contraindicated. A ¼ dose of the ARNI sacubitril/valsartan corresponds to 24/26mg bid and a ½ dose to 49/51mg bid. 3. Titration should not be considered definitive, and medication should be reassessed at each follow-up visit according to the patient's general medical status. Even in patients whose ejection fraction improves after treatment, guideline-directed medical therapy should be pursued.
ACEI, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta-blockers; CGA, comprehensive geriatric assessment; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; MRA, mineralocorticoid receptor antagonist; SBP, systolic blood pressure; SGLT2I, sodium-glucose like transporter type 2 inhibitor; TRST, Triage Risk Screening Tool; VT, ventricular tachycardia.
In addition, the presence of frailty should trigger the management of comorbidities and geriatric syndromes through a comprehensive geriatric assessment.37,45 This multidimensional assessment should take into account comorbidities, cognitive function, autonomy, walking disorders, the risk of falls, nutritional status, depression, polypharmacy, vaccination status (notably influenza, pneumococcus and COVID), and social isolation.37,45 Such multidisciplinary management has been shown to reduce mortality in older patients with HF.45 Any medical needs identified during this assessment should be addressed promptly in parallel to HF treatment. Wherever appropriate, specific comorbidities should be managed, home help (nurses and social support) and cognitive stimulation offered, physical activity adapted, and physiotherapy or psychotherapy provided as needed. Medications and vaccination status should be checked, and vitamin D supplementation, oral nutritional supplements and antidepressant treatment provided when justified. Finally, an environmental assessment should be made.
Management of patients with cardiac rhythm disordersCardiac rhythm disturbances, such as supraventricular and ventricular arrhythmias, bradycardia and conduction disturbances (mainly left-bundle branch block) are common in patients with HF, and contribute to the increased mortality and morbidity of these patients.4 For these reasons, it is important to ensure a specific diagnostic work-up for rhythm disorders and their appropriate management in all patients diagnosed with HF. Associated rhythm disorders can have an impact on the titration strategy, as in patients with ventricular tachycardia or premature ventricular contractions, BB should be introduced in the first treatment step, in preference to ACEIs or ARNIs, due to their beneficial effect on rate control.
In addition, the decision to implant a cardioverter-defibrillator should be made after drug optimization, according to ESC practice guidelines,4 in all patients with symptomatic HF (NHYA class II-III) and a left ventricular ejection fraction ≤ 35% with ischemic cardiomyopathy (Class IA recommendation) and nonischemic cardiomyopathy (Class IIa A). Rapid initiation of HF drugs is advisable to ensure timely implementation of cardioverter-defibrillators in patients in whom left ventricular ejection fraction remains ≤ 35%. In patients with HF and wide QRS and conduction disorders, cardiac resynchronization therapy should be considered, the level of recommendations depending on the QRS width and the type of conduction disorder (presence or absence of left-bundle branch block).4
Atrial fibrillation (AF) is a common comorbidity in patients with HF, its prevalence increasing with the severity of HF.46 Around half of all patients with HF either have pre-existing AF at the time of diagnosis of HF or develop AF subsequently.47 Comorbid AF may aggravate underlying HF,48 for example due to the development of tachycardia-mediated cardiomyopathy which impairs ventricular contractility.46 Patients with AF should be proposed cardioversion and antiarrhythmic drugs (limited to amiodarone in patients with reduced ejection fraction), or catheter ablation, the latter having been shown to be more effective in reducing the risk of HF exacerbation.49
HEALTHCARE ORGANIZATIONOnce the 4 classes of GDMT have been initiated, the doses will require optimization in the community setting and regular monitoring, with adjustment where necessary to avoid acute exacerbations that may be fatal and generally require rehospitalization.
Following hospital discharge after an episode of worsening HF, patients enter a vulnerable period, during which transition care programs are advocated to avoid early HF readmissions.50 A randomized clinical trial is currently underway to document the effectiveness of such programs.51
However, most HF patients are not followed up promptly, when they are still at high risk.52 The dose optimization phase is crucial and it is recommended that detailed instructions on implementation be provided in the discharge letter for patients returning home from hospital.52 Without dedicated follow-up, treatment optimization may not be implemented correctly, with more deleterious long-term effects on prognosis than the way in which the treatments were initially introduced. Unfortunately, many patients discharged from hospital never receive an optimized treatment regimen due to the inertia of the system.11,52–56 Establishing structured postdischarge follow-up is crucial, ideally through a dedicated disease management program.57,58 Trained and dedicated HF nurses are usually the cornerstone of rapid treatment optimization as they can titrate HF drugs, provide therapeutic education, ensure personalized contact with the patient and identify early any signs of deterioration. In a recent randomized clinical trial evaluating the role of nurses in up-titrating HF medication, HF nurses achieved higher doses of BB and ACEI over a 4-month period than did HF cardiologists, principally because nurses were able to see the patient much more frequently.59 In addition, multidisciplinary management involving a dedicated HF nurse has been shown to improve adherence to practice guidelines60 and clinical outcomes.61
Telemedicine programs can be especially useful to ensure timely modifications of treatment or other interventions should the patient's state deteriorate.62–64 However, telemedicine may not be appropriate for all patients, notably those with cognitive impairment or poor adherence.
Following an episode of acute decompensation, cardiac rehabilitation involving exercise training combined with psychosocial support and dietary counseling is useful for reducing the risk of rehospitalization.65 Currently, patients are not sufficiently referred to cardiac rehabilitation centers from community care.66
In primary care, delays in referral to specialist HF physicians, limited consultation time and lack of communication between health professionals can lead to inadequate implementation of optimal treatment and inappropriate follow-up.67 To avoid a silo approach to the care of HF patients in the community, multidisciplinary management involving a dedicated HF nurse and both hospital and community cardiologists is essential. To achieve this, education of health care professionals is critical and the provision of online medical expertise could be a powerful way for centers of excellence for HF care to reach out to community health care providers.
It is important to emphasize that differences in the organization of care for patients with HF clearly exist among regions and countries, and these need to be taken into account when considering how to optimize treatment pathways. However, it is also the responsibility of national decision-makers to ensure that the best quality of care can be offered to all patients and that inequalities in care provision are minimized.
CONCLUSIONSIn this position paper, we propose a pragmatic algorithm for the implementation and optimization of GDMT for the treatment of HF with reduced ejection fraction. We believe that the sequential introduction and titration of these 4 pillars of heart failure within 2 months of an acute exacerbation of pre-existing HF or a recent diagnosis can be routinely achieved in most patients with HF. This needs to be our treatment goal and every effort must be made to achieve it. The algorithm proposed above should help hospital and community physicians achieve this goal in everyday practice.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSN. Girerd and F. Roubille coordinated the manuscript. N. Girerd, C. Leclercq, O. Hanon, F. Roubille drafted parts of the manuscript. N. Girerd, C. Leclercq, O. Hanon, A. Bayés-Genís, J. L. Januzzi, T. Damy, B. Lequeux, C. Meune, P. Sabouret, and F. Roubille provided critical reviewing and editing of the initial draft.
CONFLICTS OF INTERESTF Roubille reports grants and/or personal fees from Air Liquide, Abbott, Astra Zeneca, Bayer, Boehringer, Novartis, Pfizer, Servier, Vifor; B Lequeux reports personal fees from Bayer, Boehringer, Microport, Novartis, Pfizer, Vifor; C Leclercq reports personal fees from Novartis, Medtronic, Biotronik, Microport, Abbott; P MEUNE reports grants and/or personal fees from Astra Zeneca, Novartis, Vifor, Roche Diagnostics; T Damy reports grants and/or personal fees from Novartis, Vifor, Resmed, Pfizer, Alnylam, Ionsis, Akcea, GSK, Prothena; O Hanon reports personal fees from Astra Zeneca, Bayer, BMS, Boehringer Ingelheim, Leo Pharma, Medtronic, Novartis, Pfizer, Sanofi, Servier, Vifor; P Sabouret reports personal fees from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Medtronic, MSD, Novartis, Servier, Sanofi, Vifor Pharma, and have other links of interest with Minerva Cardiology Angiology, Archives of Medical Science, Frontiers in Cardiovascular Medicine. N Girerd received personal fees from Astra Zeneca, Bayer, Boehringer, Lilly, Novartis, Pfizer, Vifor, A Bayés-Genís reports personal fees from Abbott, Novartis, Vifor, Roche Diagnostics, Critical Diagnostics and AstraZeneca, grants, personal fees and nonfinancial support from Boehringer Ingelheim. J Januzzi is a trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Abbott Diagnostics; has received consulting income from Abbott, Janssen, Novartis, and Roche Diagnostics; and has participated in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, and Takeda.
We thank Adam Doble PhD (SARL Foxymed, France) for helping to prepare the manuscript and acknowledge technical support from Novartis for illustrations.
This manuscript is also endorsed by the Heart Failure Working Group of the French Society of Cardiology.