Hypertension is a major contributor to cardiovascular events, such as stroke and myocardial infarction, with accelerated sympathetic nerve activity implicated in its pathogenesis. However, hypertension in many patients is not adequately controlled, despite the availability of numerous medication classes. Novel procedure—as well as device-based strategies, such as percutaneous renal sympathetic nerve denervation therapy—have been developed to improve blood pressure in these refractory patients. Renal sympathetic denervation delivers not only a decrease in blood pressure levels but also renal as well as systemic sympathetic nerve activity. The reduction in blood pressure appears to be sustained over 3 years after the procedure, which implies no counterregulatory mechanism or re-innervation of afferent renal sympathetic nerve so far. Renal sympathetic denervation is expected to be a promising treatment for patients with hypertension, congestive heart failure, chronic kidney disease, and metabolic syndrome implicated in the pathogenesis of potentiated sympathetic nerve activity. This review will focus on the current devices and procedures, their outcomes and prospects in the treatment of hypertension.
Keywords
Hypertension is a growing public health problem worldwide as it is a major contributor to cardiovascular diseases such as stroke and myocardial infarction.1 The estimated overall prevalence of hypertension in adults was 972 million in 2000 but is expected to increase to 1.56 billion by 2025.2 Approximately 50% of patients with hypertension do not achieve adequate blood pressure (BP) control2 as observed in studies of awareness, treatment, and control of hypertension. Adequate control of hypertension is a priority because patients with uncontrolled hypertension are at increased risk for cardiovascular mortality, doubling with each 20/10mmHg increase in BP.3 However, adequate BP levels are not obtained in many patients, despite compliance with maximum tolerance doses of >3 antihypertensive medications of different classes including a diuretic.4 This ‘resistant hypertension’ is estimated to occur in 8.9% of all adults with hypertension and 12.8% of all drug-treated hypertensive adults in the United States.5 These resistant hypertensive patients clearly need new therapeutic approaches to optimize BP control. Recently, device- and procedure-based therapies have been developed among these populations.6 A percutaneous, catheter-based renal sympathetic denervation (RND) approach has been proposed to disrupt both afferent and efferent renal sympathetic nerves initially using radiofrequency (RF) ablation (later other modalities) as a therapy for resistant hypertensive patients (Fig. 1).7
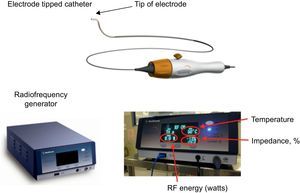
Renal sympathetic denervation system Symplicity™. There are 2 components in the Symplicity™ system: the electrode-tipped catheter and the radiofrequency generator. The electrode tip has a low profile, is flexible and self-orienting. The electrode tip delivers radiofrequency energy to the treatment site. The radiofrequency generator is applied to deliver automated and low-power radiofrequency energy to the electrode tip and to monitor the tip temperature and impedance in response to a predetermined algorithm during ablation. RF, radiofrequency. Reproduced with permission from Medtronic Inc.7
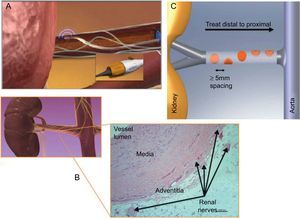
The RND procedure is safe and simple, with an average procedure time of 38min [interquartile range, 34-48min] shown in the first pilot study.6 This procedure is performed via the femoral artery with administration of heparin (initially, 5000 units: target active clotting time >250s) for anticoagulation. First, an aortogram is performed with a 5 Fr-pigtail catheter to confirm the anatomical figure of renal arteries. Once the anatomy is confirmed (appropriate diameter and length, essentially free of atherosclerotic disease), a 6 F LIMA or RDC guiding catheter is used. For the target renal artery for ablation, main renal arteries of ≥4mm in diameter and ≥20mm in length are applied. Accessory renal arteries and side branches of the main renal artery with adequate diameter and length might be considered when these are distributed >30% of kidney as a feeding artery. RF ablation at the catheter tip is then applied to the vascular wall to provide heat to the external layer and the sympathetic nerves that arborize around the artery and primarily lie within in the adventitia (Figs. 2A and B). Treatment involves circumferential coverage involving 4 to 6 treatments of low-power RF energy (8 W or less) from the distal point in both renal arteries, lasting ≤120s and administered in a spiral manner by manual rotation with approximately 5mm pullback between ablation (Fig. 2A and C). To position the catheter, the tip is placed in the most distal point of each renal artery, and then gradually deflected into the vessel wall until the electrode is well-apposed to the vessel wall. Tip temperature and impedance is monitored in response to a predetermined algorithm during ablation. Higher (∼300Ω) and stable (<20Ω in change) impedance over the respiratory cycle indicates better wall contact. A larger change in impedance indicates better delivery of energy, but abnormally high impedance and abnormally large change in impedance might suggest the electrode is in a side branch. The target reduction by impedance percentage is approximately −11% to −14%. RF ablation would be canceled when the tip temperature is ≥60°C, and the change in impedance percentage is exceedingly large.
The renal sympathetic denervation procedure is safe and simple, involving femoral artery catheterization with the tip of the catheter placed in the distal renal artery (A). For the target renal artery for ablation, main renal arteries of ≥4mm in diameter and ≥20mm in length are applied. Radiofrequency ablation at the catheter tip is then applied to the vascular wall to provide heat to the external layer and the sympathetic nerves in the adventitia (B). Treatment involves circumferential coverage for 4 to 6 treatments of low-power radiofrequency energy (8 W or less) from the distal point in both renal arteries, lasting ≤120s, and is administered in a spiral manner by manual rotation with approximately 5mm pullback between ablations (A and C). The tip temperature and impedance is monitored in response to a predetermined algorithm during ablation. Reproduced with permission from Medtronic Inc.7
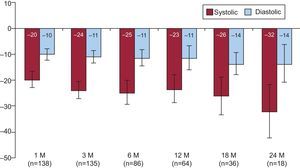
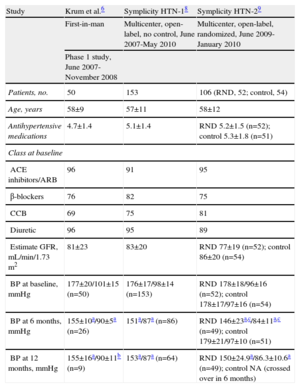
An initial first-in-man trial (Symplicity HTN-1) evaluated the efficacy and safety of this approach in patients with resistant hypertension (Table).6 The reduction of postprocedure office BPs was maintained even at 24 months’ follow-up without adverse events (reduction of 20/10, 24/11, 25/11, 23/11, 26/14, and 32/14mmHg at 1, 3, 6, 12, 18, and 24 months, respectively) (Fig. 3).8 In addition to the reduction in BP, organ-specific sympathetic denervation was demonstrated.6,9
Outcomes of Major Clinical Trials and Studies
| Study | Krum et al.6 | Symplicity HTN-18 | Symplicity HTN-29 |
| First-in-man | Multicenter, open-label, no control, June 2007-May 2010 | Multicenter, open-label, randomized, June 2009-January 2010 | |
| Phase 1 study, June 2007-November 2008 | |||
| Patients, no. | 50 | 153 | 106 (RND, 52; control, 54) |
| Age, years | 58±9 | 57±11 | 58±12 |
| Antihypertensive medications | 4.7±1.4 | 5.1±1.4 | RND 5.2±1.5 (n=52); control 5.3±1.8 (n=51) |
| Class at baseline | |||
| ACE inhibitors/ARB | 96 | 91 | 95 |
| β-blockers | 76 | 82 | 75 |
| CCB | 69 | 75 | 81 |
| Diuretic | 96 | 95 | 89 |
| Estimate GFR, mL/min/1.73 m2 | 81±23 | 83±20 | RND 77±19 (n=52); control 86±20 (n=54) |
| BP at baseline, mmHg | 177±20/101±15 (n=50) | 176±17/98±14 (n=153) | RND 178±18/96±16 (n=52); control 178±17/97±16 (n=54) |
| BP at 6 months, mmHg | 155±10a/90±5a (n=26) | 151a/87a (n=86) | RND 146±23a,c/84±11a,c (n=49); control 179±21/97±10 (n=51) |
| BP at 12 months, mmHg | 155±16a/90±11b (n=9) | 153a/87a (n=64) | RND 150±24.9a/86.3±10.6a (n=49); control NA (crossed over in 6 months) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BP, blood pressure; CCB, calcium channel blocker; GFR, glomerular filtration rate; NA, not available; RND, renal sympathetic denervation, SD, standard deviation.
Analysis is performed using the paired t-test. Data are expressed as mean±standard deviation.
An initial first-in-man trial by Krum et al.6 evaluated the efficacy and safety of the renal sympathetic denervation approach in patients with resistant hypertension. With proven safety and efficacy of the renal sympathetic denervation procedure, an extended trial, was commenced. Later, the Symplicity HTN-2 trial, a multicenter, prospective, randomized trial, was initiated.
All trials demonstrated a significant and sustained reduction in blood pressure up to 24 months.6,8–10
Blood pressure reduction after the renal sympathetic denervation procedure over 24-months of follow-up.8 Proven safety and efficacy of the renal sympathetic denervation procedure in a phase 1 study, the Symplicity HTN-1 trial, an open-label, multicenter, prospective study, was commenced. The reduction in postprocedure office blood pressures was sustained over 24 months without adverse events. M, month. Error bars represent 95% confidence intervals. Reproduced with permission from Symplicity HTN-1 Investigators.8
However, there was no control group with which to derive a comparison in the Symplicity HTN-1 trial. Therefore, the Symplicity HTN-2 trial, a multicenter, prospective, randomized trial, was performed to address this shortcoming. In this study, significant and sustained reduction in BP at 1 year follow-up was also demonstrated.10
Renal function was also evaluated by measuring the level of serum creatinine, based on estimated glomerular filtration rate. This measurement demonstrated that renal function remained unchanged over the follow-up period.6 However, albumin excretion was reduced, consistent with an overall beneficial effect on BP and possibly end-organ damage in these refractory hypertensive patients. It is also noteworthy that RND decreased renin secretion by approximately 50% and that cardiac baroreflex sensitivity improved after RND.11 In addition, cardiovascular magnetic resonance imaging revealed a substantial reduction in left ventricular mass at 12-month follow-up.11,12
These results indicate that, in addition to aggressive pharmacological therapy, RND has the potential to safely lower BP in patients with resistant hypertension. Furthermore, the sustained BP-lowering effect of RND implies that there are neither overriding alterations in counterregulatory mechanisms nor re-innervation of renal afferent sympathetic nerve after the procedure,13 at least up to 36 months thus far.
PROCEDURAL COMPLICATIONSome complications were noted during the trials, none severe. These complications included 1 hypotension episode both in the Symplicity HTN-1 trial8 and the Symplicity HTN-2 trial,9 1 renal artery dissection in the Symplicity HTN-1 trial,8 and some pseudoaneurysms/hematomas in the femoral access site, but these events were all subsequently managed without any additional complications or delays in hospital discharge.8,9
RENAL VASCULAR SAFETYNitroglycerine is often administered through a renal guide catheter to reduce arterial spasm before and after the treatment in each artery. None was considered flow limiting upon termination of the procedure. One patient in the Symplicity HTN-1 trial who underwent computed tomography angiography at 6 months postprocedure was identified as having progression of prior existing renal artery stenosis in the proximal portion of the renal artery.8 Elective angioplasty and stenting was successfully performed for this lesion.
PAINDuring RND, ablation is accompanied by sustained visceral nonradiating abdominal pain; therefore, intravenous narcotic and sedative drugs (morphine or fentanyl and midazolam) are administered in the conscious state 2-5min before the first ablation. The pain does not persist after RF ablation.
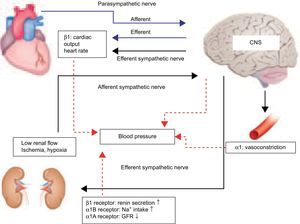
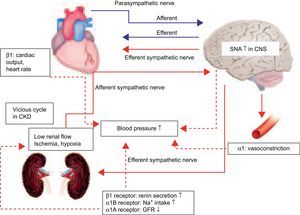
FUTURE PROSPECTS FOR RENAL SYMPATHETIC DENERVATIONSystemic BP is maintained by the autonomic nervous system, even during unconsciousness. The autonomic nervous system is made up of the sympathetic nervous system and parasympathetic nervous system (Fig. 4).14 The autonomic nervous system connects the brain, heart, blood vessels, and kidneys, regulating the maintenance of stabilized systemic BP.
Blood pressure control in autonomic nervous system. Systemic arterial pressure is controlled and maintained by the autonomic nervous system under unconsciousness. The autonomic nervous system is made up of the sympathetic nervous system and parasympathetic nervous system. The autonomic nervous system connects the brain, heart, blood vessels, and kidneys. Hypertension is induced by accelerated sympathetic nerve activity.14 Increased sympathetic nervous activity in the central nervous system is transmitted by efferent sympathetic nerves to the heart, arterioles, and kidney, thus increasing blood pressure.15 Efferent sympathetic nerves in the kidney bring sympathetic signals from the central nervous system to the kidneys, causing increased renin release, sodium retention, and reduction in renal blood flow. From the other direction, afferent nerves carry the signals from the heart, kidney, and presser organ to the central nervous system, thereby influencing sympathetic outflow to the kidneys and other organs involved in cardiovascular control. Thus, sympathetic drive creates a feedback loop that adversely affects the vasculature, heart, and kidneys, and plays a vital role in the autonomic nervous system. CNS, central nervous system; GFR, glomerular filtration rate.
When BP suddenly decreases, afferent sympathetic nerve activity (SNA) is transmitted from the heart, kidney, and pressor organs to the central nervous system (CNS) (Fig. 4). Increased SNA in the CNS initiates efferent SNA to the heart, arterioles, and kidney, which in turn contributes to the heart, arterioles, and kidney, and thus increases BP to stabilize systemic BP.15 Efferent renal sympathetic nerves bring sympathetic signals from the CNS to the kidneys, causing increased renin release, sodium retention, and reduction in renal blood flow. In contrast, when BP suddenly rises, the activity of arterial baroreceptors is transmitted by suppression to the CNS, and efferent sympathetic nerve via the CNS, and then BP is decreased to stabilize systemic BP, influencing sympathetic outflow to the kidneys and other organs involved in cardiovascular control. Furthermore, the kidney is a strong initiator of afferent SNA in the CNS but does not initiate suppressive afferent parasympathetic nerve activity to the CNS. Therefore, renal SNA is a major activator of SNA; both the contribution of the kidney to central sympathetic drive and the consequences of sympathetic efferent drive to the kidney contribute to the development and sustenance of hypertension.16 Ye et al.17,18 showed that kidney injury induced by 10% phenol increased norepinephrine secretion from the CNS and raised systemic BP in rats, but that these increases were prevented by the prior denervation of afferent renal nerves. These data suggest that information regarding the state of the kidney, such as hypertension, high glucose, ischemia, angiotensin II, and oxidative stress, is transferred via afferent renal sensory nerves to the CNS, thus stimulating efferent SNA to the heart, arterioles, and kidney, thereby elevating BP. Afferent SNA is regulated to increase BP due to low renal glomerular flow in chronic kidney disease (Fig. 5). Therefore, afferent renal SNA is activated, resulting in a decrease in renal glomerular flow via efferent renal sympathetic nerve activation, which in turn contributes to the development and maintenance of a vicious cycle of sympathetic overactivity in chronic kidney disease. Interestingly, this bidirectional relationship between sympathetic overactivity inducing insulin resistance and hyperinsulinemia producing sympathetic activation also initiates a vicious SNA cycle.19 As a consequence, RND in patients with insulin resistance or type II diabetes mellitus has the potential to improve insulin resistance and glycemic control.20 Moreover, the reduction in SNA by RND is presumed to improve cardiac dysfunction and might also prevent the development of the acute phase of heart failure in cardiorenal syndrome.
Schematic sympathetic nerve activity model of the feedback loop in chronic kidney disease. Increased sympathetic drive creates a feedback loop that adversely affects the vasculature, heart, and kidneys, and plays a vital role in the pathophysiology of hypertension. Renal sympathetic nerve activity is a major activator of sympathetic nerve activity; both the contribution of the kidney to central sympathetic drive and the consequences of sympathetic efferent drive to the kidney contribute to the development and sustenance of hypertension.16 Afferent sympathetic nerve activity in patients with chronic kidney disease is regulated to increase blood pressure due to the low renal blood flow. Therefore, afferent renal sympathetic nerve activity is activated, resulting in a decrease in renal glomerular flow via efferent renal sympathetic nerve activation, which in turn contributes to the development and maintenance of a vicious cycle to sympathetic overactivity in chronic kidney disease. CKD, chronic kidney disease; CNS, central nervous system; GFR, glomerular filtration rate; SNA, sympathetic nerve activity.
Currently, renal sympathetic nerve denervation is widespread in developed countries with expanded clinical trials. The Symplicity HTN-3 trial, designed as a prospective, randomized, masked procedure (sham operation), single blind trial, is in progress.21 New devices are also being developed22,23; one is a balloon type with RF electrodes placed in a helical pattern, which allows for directed energy flow into the adventitia, promising lower RF energy delivery and reduction in procedure time. Another is a multiablation basket with an integrated 4-point contact surface to deliver RF energy. A cylindrical transducer–catheter (PARADISETM catheter, ReCor Medical; New York, United Stated),24 emits ultrasound energy circumferentially and might be of particular benefit for RND as it does not require direct tissue contact; with a water balloon around the transducer, it passes through the surrounding fluids and generates frictional heating in soft tissues, increasing temperature at depth but with less damage to nontarget tissues. These newly developed devices for RND are expected to simplify the procedure as well as to reduce the complication risk; however, further study is needed to verify these expectations.
LIMITATIONS OF THE PROCEDURESix patients (13%) had only small BP reductions8 after RND. In these patients, the procedure might have failed, or the patients’ sympathetic efferent or afferent activity might not have contributed to their hypertension. There is also concern that predicting adequate results by RND is not possible prior to or during the procedure.
CONCLUSIONSPercutaneous, catheter-based RND represents a novel approach in the management of refractory hypertension. This procedure may also be a promising treatment for patients with less severe hypertension, congestive heart failure, chronic kidney disease, and metabolic syndrome implicated in the pathogenesis of potentiated SNA.
CONFLICTS OF INTERESTDr. Henry Krum has received research grants and consulting fees from Medtronic.
.